Martin Fleischmann
August 10, 2012
Hydrogen is the
most abundant element in the
universe. About three-quarters of the
mass of the universe is hydrogen. It's useful in much
chemistry, but
solid state physicists used a lot of hydrogen in the early
twentieth century, since it
liquefies at 14.0
K (-259.14
°C), enabling
experiments at very low
temperatures.
One perquisite of working at a
university is that you get to hear the "war stories" of the old
professors about
research in earlier days.
Dewar flasks, now made mostly from
stainless steel, were once made of
glass. A cracked Dewar would release large quantities of hydrogen gas into a
laboratory, so
explosions happened when the
hydrogen-air mixture reached an
electrical spark, usually at the
motor of a
vacuum pump.
Much of my early work involved hydrogen in
metals. At that time, there was the idea that hydrogen could be safely and inexpensively stored in metals, enabling a "
hydrogen economy." The
alloys I investigated contained
rare earth elements, which were expensive at the time. The rare earths have become much more expensive of late, as I've written in some previous articles (
Faux Palladium, January 14, 2011, and
Rare Earth Shortage, June 21, 2010).
Since I was doing
fundamental research, problems of cost didn't bother me, since the assumption was that enough research would lead to cheaper
materials. This is very often the case,
photovoltaic materials being a recent example. Since we were experienced in putting hydrogen into metals, we did research on how hydrogen would change the
magnetic and
superconducting properties of alloys. In one case, we obtained a significant result.[1]
My hydrogen work ended in late 1977, when I started my career in
industrial research. I didn't think much about hydrogen until March 23, 1989, when all my experience suddenly seemed important to many of my colleagues. On that day,
Martin Fleischmann and
Stanley Pons of the
University of Utah announced that they had fused the hydrogen isotope,
deuterium, into
helium in an
electrochemical cell.
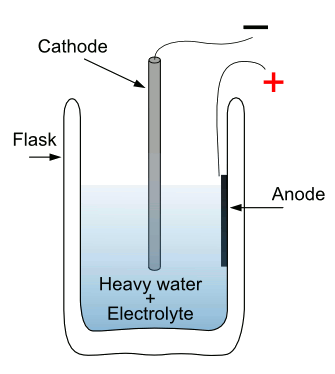
So simple. Too bad it didn't work.
This is a simplified version of the Fleischmann-Pons electrochemical cell.
Heavy water is deuterium dioxide (2H2O, but more commonly written, D2O).
(Via Wikimedia Commons))
The process, aptly named, "
cold fusion," appeared to be the answer to the world's energy problems. Although some
scientists are still doing research on the process, cold fusion was never suitably reproduced on the grand scale reported by Fleischmann and Pons. Martin Fleischmann died on Friday, August 3, 2012, at his home at
Tisbury, U.K., at age eighty-five.[2-5] A contributing cause of death was
Parkinson's disease.[3]
Apparently, this blog is one of the few places that you'll read about Fleischmann's death.[2] He seems to have been ignored because of the failed cold fusion results, but he did inspire a lot of research on hydrogen in metals. He was also an example of how science functions.
Replication of experiments and
peer review of results are the standard by which science operates.
Martin Fleischmann was born on March 29, 1927, in
Czechoslovakia, and his family emigrated to
England in 1938 to escape the
war. Fleischmann received a
Ph.D. in 1950 from
Imperial College London. When you subtract dates, it appears that he was precocious, indeed. Fleischmann was well regarded as an
electrochemist, and he was
chairman of the
chemistry department of the
University of Southampton and a
Fellow of the Royal Society. Fleischmann was awarded the
Royal Society Chemistry medal for
electrochemistry and
thermodynamics in 1979, and the
Olin-Palladium Medal from the
Electrochemical Society in 1985,[4,5]
In 1989, Fleischmann was in his sixties and chairman of the
University of Utah Chemistry Department.[4] It's reported that he and Pons invested $100,000 of their own money over a five year period in their experiments.[3] Their apparatus, shown in a simplified version in the above figure. The
anode was a coil of
platinum wire, and the
cathode was a
palladium rod. Deuterium went to the cathode, where it was absorbed by the palladium, and
oxygen went to the anode.[3]
The idea was that all those deuterium atoms squeezed into the palladium would have infrequent encounters in which they would fuse, releasing energy. The first problem was that if it worked, Fleischmann and Pons would have died from
neutron radiation. Nonetheless, many research groups, including
Los Alamos National Laboratory, tried to replicate the experiment.[2] Replication was difficult, since the Fleischmann and Pons process was not described in a research publication. There was only a
press conference and
press release.
At the time, my colleagues and I talked about buying some palladium as an investment, but none of us ever did. Surprisingly, there was no uptick in the price of palladium in that period.[6]
General Electric entered a collaborative research agreement with the University of Utah, and the
Utah State Legislature released $5 million to fund a National Cold Fusion Institute.[4] The experiments done to replicate the process disproved the deuterium fusion hypothesis, and nothing of commercial significance was evident.[7] It was a disaster for all involved.[2]
Fleischmann and Pons were criticized for their "science by press release."[3] They ignored the peer-review process that's essential to science.
Gary Taubes, in his 1993 book, "
Bad Science," wrote that the premature announcement was forced on the duo by
academic politics. Fleischmann was a good scientist who got trapped in a bad situation.[4]
At the end of 1989, things were looking grim for cold fusion. A group of scientists was recruited as an Energy Research Advisory Board, and they were commissioned to prepare a report to the
United States Department of Energy. Quoting from this November, 1989, report (DOE/S-0073 DE90 005611),[7]
"The Panel concludes that the experimental results on excess heat from calorimetric cells reported to date do not present convincing evidence that useful sources of energy will result from the phenomena attributed to cold fusion. In addition, the Panel concludes that experiments reported to date do not present convincing evidence to associate the reported anomalous heat with a nuclear process."
When the Utah National Cold Fusion Institute closed its doors, Fleischmann and Pons went to
France, where a subsidiary of
Toyota funded their further research for about $20 million through 1997.[2,4] Pons, who stopped working in cold fusion after that, is reported to be well, teaching mathematics and chemistry in the south of France.[5]
There are still a few cold fusion believers, and a there's a theory that
nuclear weak interactions might be involved in the net energy output seen in some experiments.[4] The amount of
heat liberated is very small, and errors can occur from many sources. Even the
entropy change in the material as it absorbs deuterium is important. Much of this research is now labeled as studies of "low-energy nuclear reactions" to disassociate it from cold fusion's bad image.[2]
References:
- P. Duffer, D.M. Gualtieri, and V.U.S. Rao, "Pronounced Isotope Effect in the Superconductivity of HfV2 Containing Hydrogen (Deuterium)," Phys. Rev. Lett., vol. 37, no. 21 (November 22, 1976), pp. 1410-1413.
- D. Chandler, "World Renowned Scientist, Dr. Martin Fleischmann Dies With Questionable Coverage," Guardian Express, August 8, 2012.
- Colin Schultz, "The Man who 'Discovered' Cold Fusion Just Passed Away," Smithsonian, August 8, 2012.
- Brooke Adams, "Martin Fleischmann, co-discoverer of 'cold fusion,' is dead," The Salt Lake Tribune, Augist 6, 2012.
- Steven B. Krivit, "Fleischmann Dead at 85: End of an Era," New Energy Times, August 4, 2012.
- Henry E. Hilliard, "Metals Prices in the United States through 1998--Platinum-Group Metals," USGS Minerals Information Team
- Energy Research Advisory Board, "A Report of the Energy Research Advisory Board to the United States Department of Energy," DOE Report DOE/S-0073 DE90 005611, November 1989.
- Martin Fleischmann, University of Southampton Web Site, August 8, 2012.
Permanent Link to this article
Linked Keywords: Hydrogen; abundance of the chemical elements; universe; mass; chemistry; solid state physics; twentieth century; liquefaction of gases; Kelvin; Celsius; experiment; temperature; university; professor; research; Dewar flask; stainless steel; glass; laboratory; explosion; hydrogen safety; hydrogen-air mixture; electrical spark; electric motor; vacuum pump; metal; hydrogen economy; alloy; rare earth element; pure research; fundamental research; material; photovoltaic; magnetism; superconductivity; research and development; industrial research; Martin Fleischmann; Stanley Pons; University of Utah; deuterium; helium; electrochemical cell; heavy water; Wikimedia Commons; cold fusion; scientist; Tisbury, U.K.; Parkinson's disease; reproducibility; replication of experiments; peer review; Czechoslovakia; England; World War II; Doctor of Philosophy; Ph.D.; Imperial College London; electrochemistry; electrochemist; chairman; chemistry department; University of Southampton; Fellow of the Royal Society; Royal Society; thermodynamics; Olin-Palladium Medal; Electrochemical Society; University of Utah Chemistry Department; anode; platinum; cathode; palladium; oxygen; neutron radiation; Los Alamos National Laboratory; press conference; press release; General Electric; Utah State Legislature; Gary Taubes; Bad Science: The Short Life and Weird Times of Cold Fusion; academia; academic; politics; United States Department of Energy; France; Toyota; nuclear weak interaction; heat; entropy.