Sand Battery
October 10, 2022
An
idiom that's not often heard today, since it's been replaced by more
vulgar expletives, advises someone to
pound sand. This idiom has been around since 1857, and the idea is that pounding sand is a
futile activity, since the end result is still the same sand. For that reason, it's an activity that's appropriate for someone who is not
skilled for meaningful
work.[1]

Three wise monkeys sand sculpture at Barceloneta Beach, a district of Barcelona, Catalonia, Spain.
The three wise monkeys represent a Japanese proverb, "see no evil, hear no evil, speak no evil."
They are so much a part of popular culture that they have their own emoji symbols: U+1F648 for See-No-Evil, U+1F649 for Hear-No-Evil, and U+1F64A for Speak-No-Evil.
damp sand, such as that used in the construction of sand sculptures, exhibits cohesion because the surface tension of water "glues" the sand grains together.[2]
(Wikimedia Commons image by Simon James.)
There's a fundamental
principle involved with the
idea that
manually pounding beach sand with a
hammer will have little affect. That's because
material fracture is aided by
microscopic cracks that
amplify the
force of the hammer whack, a process that was elucidated in 1921 by
aeronautical engineer,
Alan Arnold Griffith (1893-1963).[3]
Griffith conducted
experiments on
freshly-drawn glass fibers in which he added controlled flaws,
notches at their
surface.[3] Such notching greatly decreased the force needed to break the glass. The
erosion forces of
water over
geological time scales produce particles of beach sand that have already been broken at their weakest points and
polished by
wave action. As a consequence, grains of beach sand have fewer surface cracks; so, your sand pounding does not have enough force to produce many smaller particles.
As I wrote in a
previous article (Sand, October 18, 2021),
sand is one of the most
mined materials, used for making
concrete and the
silicon needed for making
integrated circuits. Inland sand is generally composed of
silica (silicon dioxide, SiO2) created from eroded
quartz, and beach sand is generally
calcium carbonate (CaCO3) created from
aragonite. Sand is a non-
renewable resource, and about 50 billion
tons of sand and gravel are used
annually in
construction.[4-5]
Sand is the enabling media for a
laboratory apparatus called a
sand bath. Sand baths have a long
history, having been used by the
alchemists, the
progenitors of
modern chemistry. A sand bath is essentially a
heated bucket of sand intended to hold and heat a buried
reaction vessel. A reaction vessel placed on a
hot plate, or above a
Bunsen burner, will be heated non-uniformly from the bottom, a sand bath heats the entire surface of the vessel. In a
heated bath, water or
oil is used in place of sand.
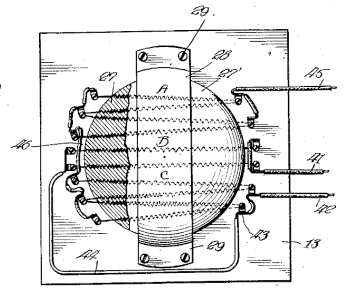
A laboratory sand bath, fig. 2 of US Patent No. 1,378,474, "Sand Bath," by Lidberg Tiodolf, May 17, 1921.
As stated in the patent text, "In using the device sand is usually placed in the bowl and any object to be heated is placed in the sand. Thus test tubes, instruments and the like may be readily and quickly brought to the desired temperature."
(Via Google Patents. Click for larger image.)
Sand baths are effective, since sand has a high
heat capacity; that is, sand at a given temperature will maintain nearly that same temperature as heat is removed or replenished. This is what a
chemist would prefer while running a reaction. Silica sand, SiO
2, has a heat capacity of 0.72436
J/
g/
K at 100°C, which is smaller than that of
liquid water at 100°C, 4.1813 J/g/K. However, silica is 2.648 times
denser than water; so, in terms of
volumetric heat capacity, we have 1.918 J/
cc/K for silica
vs 4.1813 J/cc/K for water. Also, water turns to
steam at 100°C, but silica stays
solid up to its
melting point of 1,713 °C, at which temperature the heat capacity is 1.23640 J/g/K and about 3.27 J/cc/K.
The heat capacity of SiO
2 varies quite a bit with temperature around
room temperature. Its value is given by a
polynomial approximation called the
Shomate equation,
Cp = A + Bt + Ct2 + Dt3 + E/t2
in which
Cp is the heat capacity under constant
pressure in units of J/
mol/K,
t is the
Kelvin temperature
T divided by a thousand (
t = T/1000),
A = 72.77482,
B = 1.293543,
C = -0.004360,
D = 0.000798, and
E = -4.140645. Conversion to units of J/g/K is accomplished by dividing by the
molar mass of
SiO2, 60.08 g/mol, as shown in the
graph.
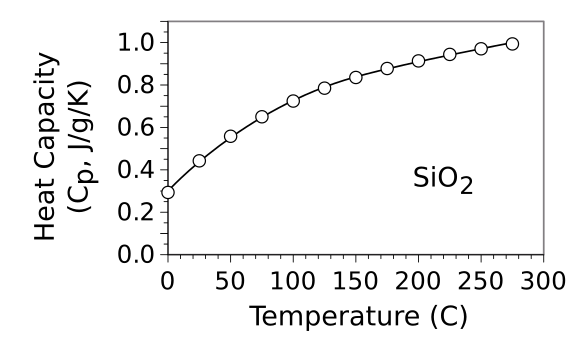
Heat capacity of SiO2 in units of J/g/K. The behavior of the heat capacity, linear at low temperatures and limiting asymptotically at high temperatures, is explained by the Debye model. This model has a temperature, called the Debye temperature, that specifies the turnover from a linear to a limited response to temperature. The Debye temperature for SiO2 is 470 K (196.85 °C). (Created using Gnumeric from the fitting parameters contained in ref. 6 (see above).[6] Click for larger image.)
The heat capacity of sand has been proposed as a way to
store energy from transient energy sources, such as
solar and
wind power, in parallel efforts by the
National Renewable Energy Laboratory (NREL), a
national laboratory of the
U.S. Department of Energy, and
engineers at
Polar Night Energy in
Finland.[7-9] The NREL is in final development of its silica sand thermal energy storage concept that can serve as a
reliable, cost-effective, and
scalable energy storage
technology that can be sited anywhere.[7]
The NREL device operates by using
surplus electricity from
renewable energy sources to heat
particles of silica sand
flowing through an
array of
resistance heating elements that heat them to 1,200°C. These heated particles fall into
insulated concrete storage
silos.[7] The baseline system can store an amazing 26,000
megawatt-hours of thermal energy in a design that can be easily scaled up or down as required.[7]
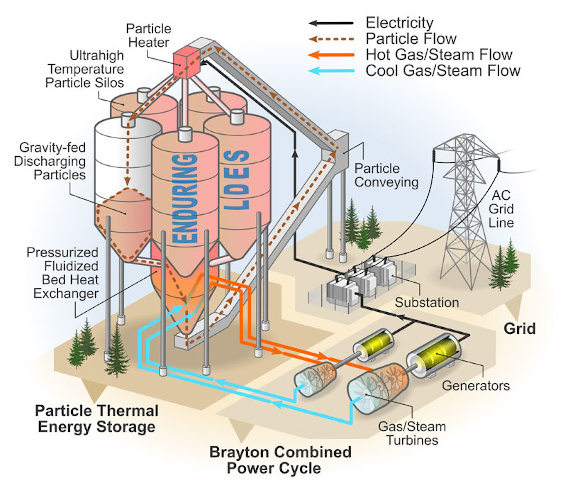
Go big or go home - The National Renewable Energy Laboratory (NREL) Sand Battery. In this particle thermal energy storage system, silica particles are gravity-fed through resistance heating elements, and the heated particles are stored in insulated concrete silos. When electrical power is needed, these heated particles are fed through a heat exchanger to drive a generator to create electricity. The operation is described by the Brayton cycle, a thermodynamic cycle in which air, or another gas, is used as the working fluid in a heat engine. (NREL image by Patrick Davenport and Al Hicks. Click for larger image.)
To extract the saved energy, the hot particles fall through a heat exchanger to heat and
pressurize a
working gas that drives a
turbo generator to create electricity, albeit at lower
energy conversion efficiency than a
lithium battery.[7] After the heat is extracted from the particles, they're returned to the insulated silos for storage until heating is again
economically feasible.[7] Although not that efficient, the system is inexpensive. Silica sand is abundant and costs just $30-$50 per
US ton; and, unlike lithium batteries, the sand lasts essentially forever.[7] Siting can be anywhere, and
decommissioned coal-fired and
gas-fired power plants can be converted to sand energy storage very easily.[7] In addition to providing electrical energy storage and heat for buildings, the system can be a heat source for
industrial and
chemical processes.[7]
The Finnish engineers are already operating the world's first sand battery in
Kankaanpää municipality capable of storing wind and solar power for months at a time.[8] The operating temperature, 500°C, for this sand battery having a hundred
tonnes (110.2 US tons) of sand in a silo is lower than that of the NREL device.[8] The device is installed in the local power plant for the district heating system for the Kankaanpää area to
homes,
offices, and a
swimming pool.[8] The operating principle, in which sand transfer heat to air in a heat exchanger, is the same as for the NREL device.[8] According to reports, this first
commercial sand battery system is working well.[8]
References:
- Pounding Sand, The Word Detective.
- The Physics of Sandcastles, NASA Website, July 11, 2002.
- A.A. Griffith, A. A. (1921), "The phenomena of rupture and flow in solids," Philosophical Transactions of the Royal Society of London, vol. A221 (1921), pp. 163–198. Available here, also.
- Vince Beiser, "Why the world is running out of sand," BBC, November 18, 2019.
- Mette Bendixen, Lars L. Iversen, Jim Best, Daniel M. Franks, Christopher R. Hackney, Edgardo M. Latrubesse, and Lucy S. Tusting, "Sand, gravel, and UN Sustainable Development Goals: Conflicts, synergies, and pathways forward," One Earth, vol. 4, no. 6 (August 20, 2021), pp. 1095-1111, https://doi.org/10.1016/j.oneear.2021.07.008.
- Silicon oxide, NIST Chemistry WebBook, SRD 69, NIST Website.
- NREL Options a Modular, Cost-Effective, Build-Anywhere Particle Thermal Energy Storage Technology, NREL Website, August 30, 2021.
- Matt McGrath, "Climate change: 'Sand battery' could solve green energy's big problem," BBC, July 4, 2022.
- NREL thermal systems and concentrating solar power research website.
Linked Keywords: Idiom; vulgarity; vulgar; profanity; expletive; pound sand; futile; skilled; work (human activity); three wise monkeys; sand art and play; sand sculpture; La Barceloneta, Barcelona; Barceloneta Beach; Catalonia; Spain; Japan; Japanese; proverb; visual perception; see; evil; hearing; hear; speech; speak; popular culture; emoji symbol; moisture; damp; sand; cohesion (chemistry); surface tension; water; crystallite; grain; Wikimedia Commons; Simon James; physical law; principle; idea; manual labor; manually; hammer; pounding; beach; fracture mechanics; material fracture; microscopic scale; crack; stress concentration; amplify; stress (mechanics); force; aerospace engineering; aeronautical engineer; Alan Arnold Griffith (1893-1963); experiment; glass fiber; notch (engineering); surface; erosion force; water; geological time scale; pPolishing; polished; wind wave; wave action; mining; mined; material; concrete; silicon; integrated circuit; silica (silicon dioxide, SiO2); quartz; calcium carbonate (CaCO3); aragonite; renewable resource; ton; year; annually; construction; laboratory equipment; laboratory apparatus; sand bath; history; alchemy; alchemist; progenitor; modern chemistry; heat; heated; bucket; chemical reactor; reaction vessel; hot plate; Bunsen burner; heated bath; oil; US Patent No. 1,378,474, 'Sand Bath,' by Lidberg Tiodolf, May 17, 1921; laboratory; patent; test tube; instrument; temperature; Google Patents; heat capacity; joule; gram; kelvin; liquid; density; denser; volume; volumetric; cubic centimeter; cc; steam; solid; melting point; room temperature; polynomial; approximation; pressure; mole (unit); mol; molar mass; Cartesian coordinate system; graph; linearity; linear; limit (mathematics); limiting; asymptote; asymptotically; Debye model; Gnumeric; nonlinear regression; fitting parameter; energy storage; solar energy; wind power; National Renewable Energy Laboratory (NREL); national laboratory; United States Department of Energy; engineer; Polar Night Energy; Finland; reliability engineering; reliable; scalability; scalable; technology; excess supply; surplus; electricity; renewable energy source; particle; fluid dynamics; flow; array; resistance heating element; thermal insulation; insulated; concrete; silo; megawatt-hour; go big or go home">Go big or go home; thermal energy storage system; gravitation; gravity; heat exchanger; turbo generator; Brayton cycle; thermodynamic cycle; atmosphere of Earth; air; gas; working fluid; heat engine; Patrick Davenport and Al Hicks; pressure; pressurize; energy conversion efficiency; lithium battery; economics; economically; US ton; decommission; decommissioned; coal-fired power station; gas-fired power plant; industrial process; chemical process; Kankaanpää; municipality; tonne; home; office; swimming pool; commerce; commercial.