Colloidal Pre-Assembly
July 18, 2022
Father's Day, founded in
1910, two
years after
Mother's Day, was
Sunday, June 19, 2022, this year. That day reminded me of some duties of
fatherhood beyond
bringing home the bacon (with due
apology to
working mothers). One of my
Christmas Eve tasks was
assembly of
children's toys, many of which had difficult to understand
assembly instructions. Assembly was facilitated by
fortified eggnog and some
logical reasoning.

Some assemblies are more difficult than others.
NASA's Perseverance Rover at a payload servicing facility at NASA's Kennedy Space Center on February 14, 2020.
(Wikimedia Commons image. A larger image (PIA23768) can be found on the NASA-JPL website here.)
While
self-assembly of
dollhouse-size objects is something that's only seen in a
Jetson's cartoon,
chemists and
crystal growers have been performing
atomic and
molecular self-assembly for more than a
century. One early example is the
Langmuir–Blodgett film, a
creation of
Nobel Chemistry Laureate,
Irving Langmuir (1881-1957), and
physical chemist,
Katharine Blodgett (1898-1979), successfully used as an
antireflection coating on
glass.
Nanoparticles can be induced to assemble into specific
shapes through the use of
electric and
magnetic fields. One example of this is
ferrofluid, a
colloidal liquid of
nanoscale magnetic particles suspended in a
fluid such as
silicone oil.

Bluing was a common laundry item in the early to mid-20th century, but it's probably hard to find today. It added a slight blue tint to white fabrics that enhanced their appearance.
Laundry bluing is is a colloid of ferric ferrocyanide (Fe4[Fe(CN)6]3). My mother used it in the 1950s, and it was one of the chemicals in my home laboratory experiments.
Bluing can be used as a reagent for the creation of magnetic nanoparticles of ferric oxide (Fe2O3),[1] and these can be used to create a ferrofluid.
(Photograph of a bottle of Mrs. Stewart's Bluing at the Edmonds Historical Museum (Edmonds, Washington), a Wikimedia Commons image by Joe Mabel.)
A recent
open access article in
Science Advances by
physicists,
chemists and
engineers at the
University of Amsterdam (Amsterdam, Netherlands), the
Delft University of Technology (Delft, the Netherlands), the
University of Michigan (Ann Arbor, Michigan), the
University of Pennsylvania (Philadelphia, Pennsylvania), and
Queen's University (Kingston, Ontario, Canada) describes creation of
synthetic materials from colloids of small
glass particles.[2-4]
As
alloy designers and crystal growers know, and as
phase diagrams assure us, atoms should assemble in just one way at a particular
pressure and
temperature condition. It is possible to
cheat by adding another factor, such as
stress in a
thin film, to produce another
phase or
properties that are significantly different from a
bulk material. In this way, the creation of
strained silicon layers results in a
semiconductor with increased
electron mobility that enables faster
transistor. Novel materials could be created from
deposition of pre-assembled
clusters, thereby bypassing the
thermodynamic forces that insist that atoms and molecules are deposited in just one way.
The challenge is to decouple the final material structure from its building blocks, and the approach taken in this study is preassembling clusters that are different from the bulk material and assembling these into a final material.[2] The fundamental problem that was overcome in this approach is that
shape is a strong determinant of structure.[2] In their experiments, the
researchers showed that they could induce colloidal shape-
anisotropic building blocks to arrange into structures that are not found in bulk self-assembly.[2]
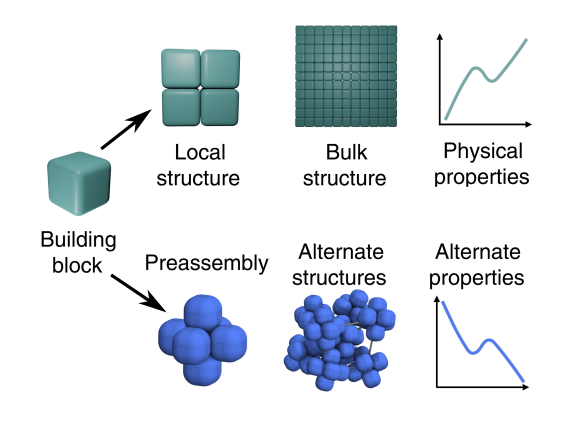
Schematic representation of the creation of a material by the usual techniques (upper steps), and from pre-assembled building blocks (lower steps). (Fig. 1 of ref. 2,[2] distributed under the Creative Commons Attribution License 4.0.)
As study
co-author,
Laura Rossi, an
assistant professor at the Delft University of Technology, explains,
"Under certain circumstances colloids can behave like atoms and molecules, but their interactions are less strong... That makes them promising building blocks for new materials, for example for interactive materials that can adapt their properties to their environment."[4]
Normally, the
cube-shaped glass colloids used in this study would assemble themselves into expected simple
lattice structures as dictated by their shapes.[4] However, when you assemble small groups of these colloids and combine these, they assemble into a different final structure with different material properties.[4] Says Rossi, "...We've shifted our focus to: how can we use the colloids that are already available to make interesting building blocks?"[4]
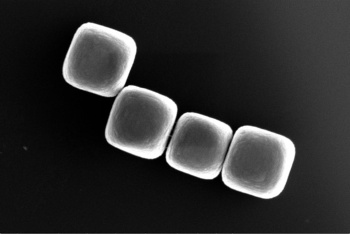
Four cubic colloids made from glass.
(TU Delft image, donated to the Public Domain.)
The research team used a technique of shape-controlled
synthesis and
emulsification to confine small numbers of glass colloid particles into clusters in which their arrangement is different than what's expected for bulk synthesis.[2] In experiments validated by
computer simulations at Queen's University, they found that
compressing colloidal
balls with different degrees of
sphericity in
spherical confinement would generate
reproducible clusters, and the structure of these are remarkably different from the bulk.[2]
Computer simulations showed that clusters of six to nine particles would assemble into a material with a structure that's different from that produced by bulk assembly of similar particles.[2] One thing that they found was that the
density of these structures was much lower than the density for bulk assembly,[4] and this might have application for variation of the
refractive index for production of
planar lenses.

scanning electron micrograph images of clusters prepared using magnetic (top row) and nonmagnetic (bottom row) particles. The configurations depend only on the number of constituent particles.
(Fig. 5b of ref. 2., distributed under the Creative Commons Attribution License 4.0. Click for larger image.)
References:
- Radek Zboril, Libor Machala, Miroslav Mashlan, and Virender Sharma, "Iron(III) Oxide Nanoparticles in the Thermally Induced Oxidative Decomposition of Prussian Blue, Fe4[Fe(CN)6]3," Crystal Growth & Design, vol. 4, no. 6 (October 5, 2004), pp. 1317-1325, https://doi.org/10.1021/cg049748+.
- Lucia Baldauf, Erin G. Teich, Peter Schall, Greg van Anders and Laura Rossi, "Shape and interaction decoupling for colloidal preassembly," Science Advances, vol. 8, no. 21 (May 27 2022), DOI: 10.1126/sciadv.abm0548. This is an open access publication with a PDF file here.
- Supplementary Materials for Ref. 2.
- New route to build materials out of tiny particles, Delft University of Technology Press Release. May 27, 2022.
Linked Keywords: Father's Day (United States); 1910; year; Mother's Day (United States); Sunday; fatherhood; bringing home the bacon; apology (act); working parent; working mother; Christmas Eve; assembly; child; children; toys; owner's manual; assembly instructions; fortified beverage; eggnog; logical reasoning; NASA; Perseverance Rover; payload; servicing facility; Kennedy Space Center; Wikimedia Commons; self-assembly; dollhouse; The Jetsons; Jetson's cartoon; chemist; crystal growth; crystal grower; atom; atomic; molecule; molecular; century; Langmuir–Blodgett film; invention; creation; Nobel Prize in Chemistry; Nobel Chemistry Laureate; Irving Langmuir (1881-1957); physical chemistry; physical chemist; Katharine Blodgett (1898-1979); anti-reflective coating; antireflection coating; glass; nanoparticle; geometry; shape; electric field; magnetic field; ferrofluid; colloid; colloidal; liquid; nanoscopic scale; magnetic; nanoscale; particle; suspension (chemistry); suspended; fluid; silicone oil; bluing (fabric); laundry; early to mid-20th century; blue; white; textile; fabric; Prussian blue; ferric ferrocyanide (Fe4[Fe(CN)6]3); mother; 1950s; chemical compound; chemical; home; laboratory; experiment; reagent; Edmonds Historical Museum (Edmonds, Washington); Mrs. Stewart's Bluing; Joe Mabel; open-access journal; open access article; Science Advances; physicist; chemist; engineer; University of Amsterdam (Amsterdam, Netherlands); Delft University of Technology (Delft, the Netherlands); University of Michigan (Ann Arbor, Michigan); University of Pennsylvania (Philadelphia, Pennsylvania); Queen's University (Kingston, Ontario, Canada); chemical synthesis; synthetic; material; glass; alloy; designer; phase diagram; pressure; temperature; cheating; cheat; stress; thin film; phase (matter); material properties; bulk; strained silicon; semiconductor; electron mobility; transistor; thin film deposition; cluster; thermodynamics; thermodynamic; research; researcher; anisotropy; anisotropic; schematic representation; technology; technique; Creative Commons Attribution License 4.0; co-author; Laura Rossi; assistant professor; interaction; environment (biophysical); cube-shaped; lattice; cube; cubic; colloid; emulsion; emulsification; computer simulation; compression (physical); compressing; sphere; ball; sphericity; spherical; reproducibility; reproducible; density; refractive index; plane (geometry); planar; lens (optics); scanning electron micrograph.