Tungsten
February 8, 2021
Civilization has been shaped by its
materials; so much so, that some of the
Ages of Man have been named by the materials used in those periods. The first such age, the
Stone Age, had a duration of about three million years.
Gold and
silver were the first useful
metals, but
archaeologists don't have a
Gold Age or a
Silver Age in their
chronology. The advent of
copper metalworking gave us the
Chalcolithic Age, followed by the
Bronze Age.

A Stone Age mortar and pestle, c. 20,000 BC.
Stone is quite abundant, and many types of stone have mechanical properties that are sufficient for stone knives and spear heads.
This item, now at the Israel Museum (Jerusalem, Israel), stands about 29 cm high and weighs 11 kilograms (24.25 pounds).
(Wikimedia Commons image by Gary Todd
The Bronze Age was the first
alloy age, since bronze is an alloy of copper with
tin. Alloying with tin gives copper greater
hardness and
strength. Bronze exists over a range of tin
concentrations, but modern
bronze generally contains about 10% tin. At about the
12th century BC, the Bronze Age was followed by the
Iron Age, the last
Age defined by archaeologists. Iron is an
abundant element, but it's hard to
separate from its compounds. Its
melting point, 1,538
°C (2,800
°F) is much higher than that of copper, 1,085 °C (1,985 °F).
The archaeological ages persist only until the advent of
written history. For that reason, the Iron Age ended during the first few centuries BC as regional histories were first written. We still use a lot of iron, today; so, it can be argued that we are still living in the Iron Age. While present
society appears to be dominated by
plastic, if we were to name it as a
metal age, it would definitely be the
Aluminum Age. Aluminum, like iron, is an abundant element, but it has advantages over iron in being
lightweight, and
corrosion-resistant. It was a latecomer to widespread use since, like iron, it is difficult to
extract from its ores.
The production of iron is by the
reduction of
iron oxide to pure iron, but
aluminum oxide is much, much harder to reduce to produce aluminum. That's because aluminum oxide is a far more stable compound than iron oxide. At 1000 K, the
free energy of formation (ΔG
f) of
Fe2O3 is -134.082
kcal/
mole, and the free energy of formation of
Al2O3 is -325.397 kcal/mole, the negative signs indicating an
exothermic reaction.[1]
The element, aluminum, was discovered in 1824 by
Hans Christian Ørsted (1777-1851), for whom the
magnetic field unit, the
oersted, is named, but our
Aluminum Age didn't begin until 1886 with the
invention of an
electrochemical process for the extraction of aluminum from
molten cryolite (Na
3AlF
6). Application of an
electric current through cryolite using a
carbon cathode and
anode creates
liquid aluminum at the cathode.
Recycling of aluminum is important, since about 25-30
kilowatt-hours of
electricity are required to produce two
kilograms of aluminum. This is about the daily electrical consumption of a typical US
home.

Master Mechanic, P. H. McLaughlin, fitting the aluminum apex on the Washington Monument, 1884.
In 1884, aluminum was as valuable as silver (about $25/ounce in today's money), and the Washington Monument was topped with an aluminum apex in that year.
This 2.85 kg apex was about 97.5% aluminum with some alloying elements, and it was intended as a lightning rod.[2]
The electrochemical process for refining aluminum, the Hall–Héroult process, was independently invented by American chemist, Charles Martin Hall and French inventor, Paul Héroult. Hall founded the company that would become Alcoa in Pittsburgh in 1888.
(Harper's Weekly illustration by S.H. Nealy, United States Library of Congress Prints and Photographs division digital ID cph.3b44599, via Wikimedia Commons.)
Early in 2020,
materials scientist,
Ainissa Ramirez, who is also host of the
Science Underground podcast, wrote about how another useful element,
tungsten, was tamed to submission by a
General Electric physicist,
William D. Coolidge (1873-1975).[3] Tungsten, as most people know, is the material that made possible the production of long-lived
incandescent light bulbs that initially enabled our
nocturnal lifestyle.
Incandescent light bulbs have been supplanted after a nearly
century's long dominance by
energy efficient LED lighting. Coolidge also advanced
X-ray tube technology through the use of a tungsten
filament and the
rotating anode X-ray tube that allowed generation of X-rays of
high power. He became
director of the
General Electric Research Laboratory in 1932, and a GE
vice president from 1940-1944.
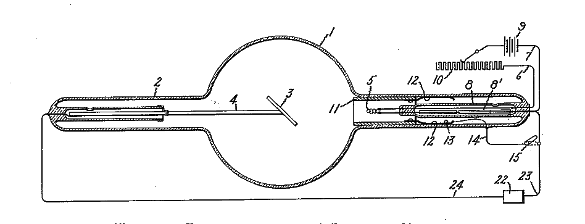
Figure 1 from US Patent No. 1,203,495, 'Vacuum Tube,' by William D. Coolidge, October 31, 1916 (Via Google Patents.[4])
The quality of light from an incandescent light bulb depends critically on the
temperature of the glowing filament. A filament at 1000°C will glow orange, but white light emission requires a much higher temperature of 1300°C (see figure). The first light bulb filaments were made from
carbon filaments, and the best such filaments, derived from
carbonized bamboo, could function for several hundred hours. In seeking hotter, brighter, and whiter, light,
manufacturers tried using high
melting point metals such as
osmium,
tantalum, and tungsten.[3] Tungsten had all the proper properties for a filament, but it was nearly impossible to process into a fine
wire.[3]
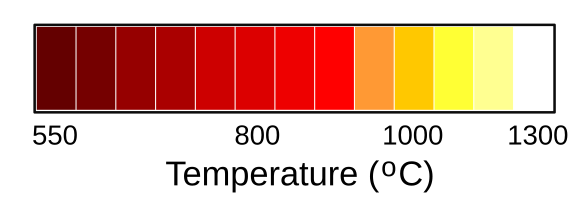
Colors associated with incandescent temperatures. The temperature scale is not linear. There will be a slight variation from material to material, since filaments are not ideal black bodies. (Modified Wikimedia Commons image by RaySys. Click for larger image.)
For those who think that
student debt is just a recent problem, it was one factor in William Coolidge's decision in 1905 to accept a job at General Electric offered by his former
MIT chemistry professor and first GE Research Laboratory director,
Willis Whitney (1868-1958), and leave his MIT
instructorship.[3] Whitney started
research on light bulb filaments using the
refractory metals. The term, "
refractory," was apt, since these were difficult to fabricate into items such as wire.
Coolidge first examined tantalum.[3] Tantalum is very easy to fashion into wire, and I've made high temperature
heaters from tantalum wire. A problem that Coolidge discovered was that tantalum filaments would fail quickly when excited by
alternating, rather than
direct,
current. Apparently, the repeated small movement caused by the
magnetic field reversal in the wire from AC resulted in
material fatigue that broke the filament. Coolidge then moved on to research in
molybdenum and tungsten.[3] Tungsten has the highest melting point of all the elements (3,422 °C, 6,192 °F), so it can be made to glow with a white light without coming anywhere near its melting point. However, tungsten is too hard to be made into a wire using conventional
wire drawing techniques.
Like a good materials scientist, Coolidge tried many material processing techniques in order to make tungsten wire. He mixed tungsten
powder with
organic binders, but then found that he could make an
amalgam of tungsten with
mercury,
extrude that, and then heat it to remove the mercury.[3] The tungsten filaments produced by this process worked, but they were
fragile and failed under
vibration.[3]
Finally, the winning process was a modification of the conventional
wire drawing process in which tungsten rods were made by
sintering pure tungsten powder, the rods were
swaged, and the heated rods were drawn down repeatedly through heated
dies.[5] The resultant 6
micrometer wire became the basis of a huge
market for GE, and nearly all light bulbs had tungsten filaments by 1916.[3]
References:
- L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- George J. Binczewski, "The Point of a Monument: A History of the Aluminum Cap of the Washington Monument," JOM, vol. 47, no. 11 (1995), pp. 20-25.
- Ainissa Ramirez, "Tungsten's Brilliant, Hidden History," American Scientist, vol. 108, no. 2 (March-April 2020), pp. 88-91, DOI: 10.1511/2020.108.2.88.
- William D Coolidge, "Vacuum Tube," US Patent No. 1,203,495, October 31, 1916.
- C. L. Briant, and B. P. Bewlay, "The Coolidge process for making tungsten ductile: The foundation of incandescent lighting," MRS Bulletin, vol. 20, no. 8 (August, 1995), pp. 67-73, DOI: https://doi.org/10.1557/S0883769400045164.
- Website of Ainissa Ramirez.
Linked Keywords: Civilization; material; Ages of Man; Stone Age; gold; silver; metal; archaeology; archaeologist; chronology; copper; metalworking; Chalcolithic; Chalcolithic Age; Bronze Age; Stone Age mortar and pestle; Anno Domini; BC; mechanical properties; stone tool; stone knives and spear heads; Israel Museum (Jerusalem, Israel); cm; kilogram; pound (mass); Gary Todd; alloy; tin; hardness; strength of materials; concentration; bronze; 12th century BC; Iron Age; abundant element; ancient iron production; melting point; Celsius; °C; Fahrenheit; °F; recorded history; written history; society; plastic; metal; lightweight; corrosion-resistant; mining; extraction from ore; smelting; reduction; iron(III) oxide; iron oxide; aluminum oxide; Gibbs free energies of formation; Fe2O3; calorie; kcal; mole (unit); Al2O3; exothermic reaction; Hans Christian Ørsted (1777-1851); magnetic field; oersted; invention; electrochemistry; electrochemical; melting; molten; cryolite; electric current; carbon; cathode; anode; liquid; recycling; kilowatt-hour; electricity; kilogram; home; apex; Washington Monument; silver; time value of money; today's money; solid solution strengthening; alloying element; lightning rod; electrochemical process; Hall–Héroult process; American; chemist; Charles Martin Hall; French; Paul Héroult; Alcoa; Pittsburgh; Harper's Weekly; United States Library of Congress; Wikimedia Commons; materials science; materials scientist; Ainissa Ramirez; Science Underground; podcast; tungsten; General Electric; physicist; William D. Coolidge (1873-1975); incandescent light bulb; night owl (person); nocturnal lifestyle; century; energy conversion efficiency; energy efficient; LED lamp; LED lighting; X-ray tube; incandescent light bulb filament; rotating anode X-ray tube; power (physics); high power; director (business); General Electric Research Laboratory; vice president; Google Patents; temperature; carbon filament; carbonization; carbonize; bamboo; manufacturing; manufacturer; melting point; osmium; tantalum; wire; incandescent; color; incandescence; linearity; linear; black body; RaySys; student debt; Massachusetts Institute of Technology; MIT; chemistry; professor; Willis Whitney (1868-1958); instructorship; research; refractory metal; refractory; electric heater; alternating current; direct current; electric current; magnetic field; material fatigue; molybdenum; wire drawing; metal powder; organic compound; binder (material); amalgam (chemistry); mercury (element); extrusion; extrude; brittleness; fragile; vibration; wire drawing; sintering; swaging; swaged; die (manufacturing); micrometer; market (economics).