Metal Powder Energy
January 18, 2021
I occasionally
author circuit construction articles in
hobby electronics magazines. When I
publish a circuit, I'm always careful to assure that all the
components are available for
purchase at the time of my writing; otherwise, the circuit would be nearly worthless as published or need tedious revision.
Scientific experiments have a similar problem, and it's solved by freely
sharing unique
reagents,
software, etc., so that experiments can be
reproduced.
Books and magazines were the only resources available to me as an
elementary school experimenter in the dark days before the
Internet. I would
gleefully buy one book or another about
kitchen experiments for
children, only to find that most of the
chemicals and
equipment weren't accessible to me. I had
baking soda and
vinegar, but no
lycopodium powder. In retrospect, it was probably good that I didn't have lycopodium powder, since the stuff is
dangerous.

A Lycopodium cernuum var. dussii plant (Scientific classification: Plantae, Tracheophyta, Lycopodiopsida, Lycopodiales, Lycopodiaceae, Palhinhaea, Lycopodium cernuum dussii), native to Guadeloupe.
Lycopodiopsida (clubmoss) plants produce spores that are the source of lycopodium powder
(Wikimedia Commons image by Patrice78500)
Lycopodium powder is a fine
powder which is the
dried spores of
clubmoss plants. Its attraction is its high
flammability, a consequence of the
material's having a high
surface area in contact with
air. Lycopodium powder is used to create
theatrical explosions and
special effects; and, in the past, it was used as a
flash powder by early
photographers. Because of the small
particle size, lycopodium powder can be used to demonstrate
Brownian motion, and it's much easier to obtain than the
pollen grains of
Robert Brown's discovery experiment.
Lycopodium powder was a useful
technological material, and its utility was facilitated by its extreme
hydrophobic nature that keeps the powder
dry. Lycopodium powder was used by
Chester Carlson (1906-1968) in some of his
xerography experiments. I wrote about Carlson in an
earlier article (Larry Tesler (1945-2020), March 30, 2020).
French inventor,
Nicéphore Niépce (1765-1833), who created the first
photograph in 1822, also invented the first
internal combustion engine, which he called the Pyréolophore, in 1807. This engine was
fueled by lycopodium powder.
The same principle behind the rapid
combustion of lycopodium powder is responsible for many
industrial dust explosions. The following table lists a few of the more significant of these.
| Name |
Date |
Place |
Material |
Fatalities |
| Washburn "A" Mill explosion |
May 2, 1878 |
Minneapolis, Minnesota |
flour dust |
22 |
|
| Douglas Starch Works explosion |
May 22, 1919 |
Cedar Rapids, Iowa |
corn starch |
43 |
|
| Mount Mulligan mine disaster |
September 19, 1921 |
Mount Mulligan, Australia |
coal dust |
75 |
|
| Benxihu Colliery explosion |
April 26, 1942 |
Benxi, China |
coal dust and gas |
1,549 |
|
| Harbin textile factory explosion |
March 17, 1987 |
Harbin, China |
flax dust |
58 |
|
| Imperial Sugar explosion |
February 7, 2008 |
Port Wentworth, Georgia |
sugar dust |
14 |
|
| 2014 Kunshan explosion |
August 2, 2014 |
Kunshan,China |
metal powder |
146 |

Not so sweet - Aftermath of the Imperial Sugar refinery dust explosion of 2008.
This dust explosion on February 7, 2008, at the sugar refinery at Port Wentworth, Georgia, killed 14 people.
(Wikimedia Commons image from the U.S. Chemical Safety and Hazard Investigation Board.)
The last incident listed in the table is significant, since it shows that
metals, not just
coal and
organic materials, will quickly combust when in powder form. Even a common and inexpensive metal like
iron releases a lot of
energy when
forming its
oxide, as the following
graph shows.
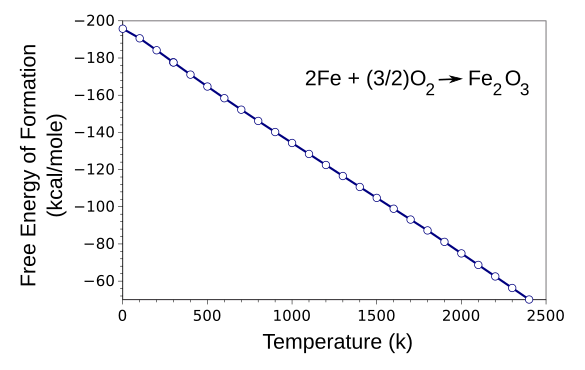
Gibbs free energy of formation of haematite (often called hematite) from the elements as a function of temperature. These data are from the JANAF Thermochemical Tables, available at the NIST Standard Reference Data Website.[1] I prefer kilocalories to joules because of my materials science background, so I converted the tabulated SI units of free energy to kilocalories. negative energy indicates an exothermic reaction. The graph was produced using Gnumeric. Click for larger image.
Researchers from the
Eindhoven University of Technology have used this energy conversion reaction as a
renewable energy source in
heat-intensive
processes at a local brewery, the
Swinkels Family Brewers,
Noord-Brabant, in a
pilot operation for
brewing 15 million
glasses of
beer.[2-5]. The
research was undertaken by
SOLID, a
multidisciplinary team of about 30
students from Eindhoven University of Technology that's been active since 2016.[6] Also participating is Metal Power, a
consortium of Noord-Brabant
companies.[2]
Heat-intensive industries are responsible for a large portion of
global carbon dioxide emissions, but the iron powder combusts without a release of
carbon dioxide.[2] The iron powder is used as a
circular fuel; that is, the oxidized iron can be
recycled into iron, and this can be done using
renewable energy sources.[2] Says
Philip de Goey, a professor at the Eindhoven University of Technology,
"The beauty of iron fuel is that you can release the energy stored in iron fuel when and where you need it... If you grind iron into a powder, it becomes highly flammable and this combustion releases a lot of energy in the form of heat. This heat can meet the industry's energy demand... No CO2 is produced during combustion and only rust remains... It's a circular process: you capture this rust powder and sustainably convert it back into iron powder."[2]
There as other advantages to iron fuel. It's
safe, no energy is lost during during storage, and it can be easily
transported.[2] One disadvantage, however, is its low
specific energy, just 1.4
kWh/
kg, so its
energy density is about an
order of magnitude less than
gasoline.[3] This means it isn't suitable for
automotive fuel, but industrial applications, such as the brewery demonstration, are possible. The combustion heat can be used also for industrial, or
residential heating.[3,7]

Combustion of iron in a combustion tube.
Since I've done experiments with molten metals, my calibrated eye sees a temperature of about 1000°C.
(Eindhoven University of Technology image by Bart van Overbeeke.)
The conversion of the iron oxide to elemental iron is by its
reaction with
hydrogen to convert the contained
oxygen to
water. This can be done in several ways, one of which is just heating the iron oxide in a hydrogen atmosphere at 800-1000°C.[3] Other methods are using a
fluidized bed reactor at the lower temperature of about 600°C for a longer time; or, a rapid method of blowing the iron powder in a stream of hydrogen at 1100-1400°C.[3] Hydrogen is thus used in a stored energy process without the problems of transporting the hydrogen itself.[3] Plans are in place for a 10 MW system in 2024.[2]
References:
- Fe2O3 (Haematite) from the NIST-JANAF Thermochemical Tables, Fourth Edition, Part I and Part II, M.W. Chase, Jr., Editor, found at NIST Standard Reference Data, Hematite (Fe2O3). Earlier data can be found in C. E. Wicks and F. E. Block, "Thermodynamic Properties of 65 Elements - Their Oxides, Halides, Carbides, and Nitrides," U. S. Bureau of Mines Bulletin 605, U. S. Government Printing Office (1963);, with an Online version, via The University of North Texas Library.
- TU/e demonstrates iron fuel at brewery Bavaria: a new circular and CO2-free fuel for the industry, Eindhoven University of Technology Press Release, October 29, 2020.
- Evan Ackerman, "Iron Powder Passes First Industrial Test as Renewable, Carbon Dioxide-Free Fuel," IEEE Spectrum, November 13, 2020.
- World's first iron-based energy storage system, YouTube Video by Solid, September 6, 2018,
- Iron Powder - the green energy solution, YouTube Video by the Eindhoven University of Technology, October 21, 2020.
- Team Solid Website, https://teamsolid.org.
- J.M.Bergthorson, S.Goroshin, M.J.Soo, P.Julien, J.Palecka, D.L.Frost, and D.J.Jarvis, "Direct combustion of recyclable metal fuels for zero-carbon heat and power," Applied Energy, Vol. 160 (December, 2015), pp. 368-382, https://doi.org/10.1016/j.apenergy.2015.09.037.
Linked Keywords: Author; electronic circuit; hobby; electronics; magazine; publishing; publish; electronic component; purchasing; purchase; science; scientific; experiment; sharing; reagent; software; reproducibility; reproduce; book; elementary school; Internet; joy; glee; kitchen; child; children; chemical compound; chemical; laboratory equipment; sodium bicarbonate; baking soda; vinegar; lycopodium powder; hazard; dangerous; Lycopodium cernuum var. dussii plant; taxonomy (biology); scientific classification; plant; Plantae; vascular plant; Tracheophyta; Lycopodiopsida; Lycopodiaceae; Lycopodiales; Palhinhaea; Lycopodium cernuum dussii; indigenous (ecology); native; Guadeloupe; clubmoss; spore; Wikimedia Commons; Patrice78500; powder (substance); Desiccation; dried; dry; flammability; material; surface area; atmosphere of Earth; air; theater; theatrical; explosion; special effect; flash powder; photographer; particle size; Brownian motion; pollen grain; Robert Brown; technology; technological; hydrophobe; hydrophobic; Chester Carlson (1906-1968); xerography; France; French; invention; inventor; Nicéphore Niépce (1765-1833); photograph; internal combustion engine; fuel; combustion; industry; industrial; dust explosion; death; fatality; Washburn "A" Mill explosion; Minneapolis, Minnesota; flour dust; Cedar Rapids, Iowa; corn starch; Mount Mulligan mine disaster; Mount Mulligan, Australia; coal dust; Benxihu Colliery explosion; Benxi, China; coal gas; Harbin, China; flax dust; Imperial Sugar explosion; Port Wentworth, Georgia; sugar dust; 2014 Kunshan explosion; Kunshan,China; metal powder; sweetness; sweet; Imperial Sugar; refinery; U.S. Chemical Safety and Hazard Investigation Board; metal; coal; organic compound; iron; Gibbs free energy; chemical reaction; formation; oxide; Cartesian coordinate system; graph; hematite; haematite; chemical element; function (mathematics); thermodynamic temperature; data; JANAF Thermochemical Tables; NIST Standard Reference Data Website; calorie; kilocalorie; joule; materials science; International System of Units; SI units; negative number; exothermic reaction; Gnumeric; Eindhoven University of Technology; renewable energy source; heat; industrial process; processes; Swinkels Family Brewers; Noord-Brabant; pilot plant; pilot operation; brewing; glassware; glass; beer; research; SOLID; multidisciplinary approach; multidisciplinary team; student; consortium; company; greenhouse gas; global carbon dioxide emissions; carbon dioxide; thermodynamic cycle; circular; recycling; recycle; Philip de Goey; milling (grinding); grind; rust; renewable resource; sustainably; safety; safe; transport; transported; specific energy; kilowatt-hour; kWh; kilogram; kg; energy density; order of magnitude; gasoline; motor fuel; automotive fuel; residence; residential; central heating; combustion; iron; chemical reactor; combustion tube; experiment; melting">molten; metal; calibration; calibrated; human eye; temperature; Celsius; °C; Bart van Overbeeke; chemical reaction; hydrogen; oxygen; water; fluidized bed reactor; Fe2O3 (Haematite).