Crystallization
May 10, 2021
Atomism, the
idea that every
substance is constructed from fundamental indivisible components, started with the
ancient Greek philosopher,
Leucippus (fl. 5th century BC). The
conjectured existence of such
particles was even considered as late as 1714, when
Gottfried Wilhelm Leibniz (1646-1716) wrote about his atomic units, the
monads, in his short
treatise,
Monadologie. Less than two
centuries later, atomism left the realm of
philosophy and entered the realm of
science.
A collection of
atoms wouldn't be anything more than a
gas unless they could join together. The Greek philosophers considered this problem, and their solution was to imagine that atoms had
hooks on the outside that allowed them to
coalesce (see figure). This
Velcro chemistry was later refined into the
theory of the
chemical bond, first by
Gilbert N. Lewis (1875-1946) in his 1916
valence bond theory, and later by
Linus Pauling (1901-1994) in his ideas of
resonant bonding and
orbital hybridization.[1] I wrote about Lewis in an
earlier article (Gilbert N. Lewis, November 16, 2011).
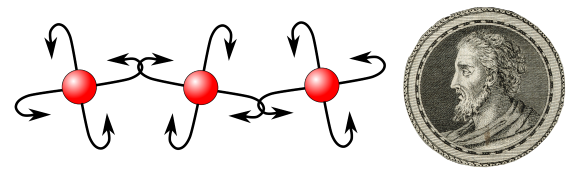
A diagram of hooked atoms and an engraving of Leucippus, originator of atomism. Is it any wonder that ancient seafarers and fishermen would consider hooks as a means to bind atoms? (Hooked atom diagram created using inkscape. Leucippus image, portion of a Wikimedia Commons image of a line engraving by S. Beyssent after Mlle C. Reyde from the Wellcome Trust, ICV no. 3727, photo no. V0003528. Click for larger image.)
Not only do atoms coalesce, but they arrange themselves into ordered structures and form
crystals. In fact, it's very difficult to produce an
amorphous (
noncrystalline)
material. This is generally done by
freezing in place the
random atomic order of a
liquid or gas by
rapid cooling. One technique to achieve amorphous materials is
splat cooling, also known as
splat quenching. Another is
melt spinning in which
molten metal is formed into a thin
ribbon of
amorphous metal by contact with a
rotating heat-sinking flywheel.
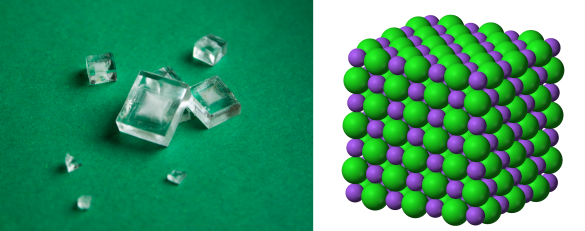
Left image, crystals of Halite (rock salt) created by slow crystallization from a concentrated salt solution at room temperature. Right image, the crystal structure of sodium chloride (NaCl) with the larger chlorine anions shown in green. (Left image; and, right image by Benjah-bmm27, both from Wikimedia Commons. Click for larger image.)
Polycrystalline materials are formed at slower
cooling rates than amorphous materials, and the small crystal
grains of polycrystalline metals can be enlarged by
annealing. In annealing, the metal is kept hot for some time to allow atoms to
migrate to more stable positions.
Simulated annealing is an
optimization technique based on this principle. Large crystals of
silicon are
grown using the
Czochralski method at a growth rate of about a
millimeter per
minute. Crystals of
neodymium-doped YAG (Nd:YAG), an important
laser material, can only be grown at about a millimeter per
hour, primarily because the atoms of not one, but several,
elements need to find their proper
lattice positions.
The dimension of a
unit cell of YAG is 1.19677
nanometers, so a growth rate of a millimeter per hour
calculates to the addition of 232 unit cells per
second; so, it takes just a little less than 5 milliseconds to add a layer of one unit cell to a growing crystal of YAG. This appears to be quite rapid, but the crystal is being formed from atoms that have a huge
velocity in the liquid. We can calculate the
mean thermal velocity of a
yttrium atom, as follows:
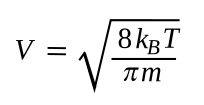
where k
B is the
Boltzmann constant (1.380649x10
−23 J/
K, in which a J/K is m
2·kg/(s
2·K) in
SI base units), T is the
absolute temperature, and m is the
mass of a yttrium atom. The
melting point of YAG is about 2250 K, and I've calculated the weight of a yttrium atom as 1.48x10
-25 kg. This leads to a yttrium atom thermal velocity in the liquid of nearly a
kilometer per second. During
crystallization, there's obviously a lot of activity at the growth
interface.
An international team of
scientists has just
published a study that examined the early stage
nucleation of crystals at the atomic level,[2-3] The team members were from
Hanyang University (Ansan, Republic of Korea), the
Korea Advanced Institute of Science and Technology (KAIST, Daejeon, Republic of Korea),
Lawrence Berkeley National Laboratory (LBNL, Berkeley, California),
Integrated Dynamic Electron Solutions, Inc. (Pleasanton, California),
Seoul National University (Seoul, Republic of Korea), the
Institute for Basic Science (IBS, Seoul, Republic of Korea),
Yonsei University (Seoul, Republic of Korea), the
University of California (Berkeley, California), and the
Kavli Energy NanoSciences Institute (Berkeley, California). Their study showed the existence of
dynamic and
reversible fluctuations of developing
nuclei between disordered and crystalline states as a
gold crystal was formed on a
graphene substrate.[2-3]
According to a
classical concept of crystal growth, crystals are formed one atom at a time.[3] Transient, high
energy arrangements of these atoms are first formed, but these atoms rearrange to the lower energy forms of a stable crystal.[3] The present study finds that atoms are involved in a dynamic and reversible fluctuation between disordered and crystalline states while building crystal nuclei.[2] In the study, gold atoms don't just group together one-by-one or make a single irreversible transition, but they will self-organize,
amorphize, and then reorganize many times on the path to their stable configuration.[3]
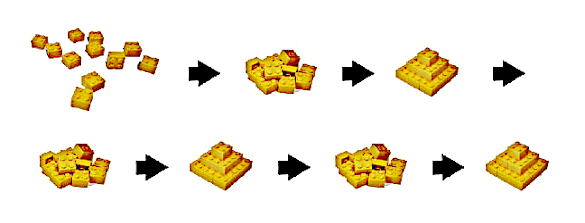
Crystal nuclei built from atoms, disordered, then built again. This is a Lego® reconstruction of an observed sequence of gold crystal formation. (Portion of a Berkeley Lab image).
It was found that the
lifetime in the disordered state decreases with increasing
atom cluster size.[2] For small nuclei, the
binding energy per atom is enough to induce melting that will cause a partial collapse of an ordered cluster into a disordered state.[2] That's because crystal formation is an
exothermic process.[3] However, when the nuclei crystals are large enough, they are locked into their ordered state.[3] The research team validated this process by
computer simulation of the binding reaction between gold atoms and a
nanocrystal.[3]
This process of the formation of gold crystals on a graphene substrate was observed through the
reduction of a
precursor by the
electron beam in an
electron microscope.[2] The electron microscope had millisecond temporal resolution that allowed observation of the rapid dynamic structural fluctuations.[2-3] It captured atomic-resolution images at speeds up to 625
frames per second, about a hundred times faster than previous studies.[3] As study
author,
Peter Ercius of Lawrence Berkeley laboratory explains,
"Slower observations would miss this very fast, reversible process and just see a blur instead of the transitions, which explains why this nucleation behavior has never been seen before,"[3]
The research team plans to view such transitions in other atomic systems to find whether it's a general process of nucleation.[3] The research was principally
funded by the
National Research Foundation of Korea. Other funding was from the
United States Department of Energy, the
Institute for Basic Science (Korea), the
Samsung Science and Technology Foundation, and the
United States National Science Foundation.[3]
References:
- Linus Pauling and The Nature of the Chemical Bond: A Documentary History - Special Collections - Oregon State University.
- Sungho Jeon, Taeyeong Heo, Sang-Yeon Hwang, Jim Ciston, Karen C. Bustillo, Bryan W. Reed, Jimin Ham, Sungsu Kang, Sungin Kim, Joowon Lim, Kitaek Lim, Ji Soo Kim, Min-Ho Kang, Ruth S. Bloom, Sukjoon Hong, Kwanpyo Kim, Alex Zett, Woo Youn Kim, Peter Ercius, Jungwon Park, and Won Chul Lee, "Reversible disorder-order transitions in atomic crystal nucleation," Science, vol. 371, no. 6528 (January 29, 2021), pp. 498-503, DOI: 10.1126/science.aaz7555.
- Clarissa Bhargava, "Revealing the Nano Big Bang – Scientists Observe the First Milliseconds of Crystal Formation," Lawrence Berkeley Laboratory Press Release, March 25, 2021.
- Revealing the Nano Big Bang in Crystal Formation, Lawrence Berkeley Laboratory YouTube video, March 24, 2021.
Linked Keywords: Atomism; idea; chemical substance; ancient Greek philosophy; ancient Greek philosopher; Leucippus (fl. 5th century BC); conjecture; conjectured; particle; Gottfried Wilhelm Leibniz (1646-1716); monad (philosophy); treatise; Monadology; Monadologie; century; philosophy; science; atom; gas; fish hook; coalescence (physics); coalesce; hook and loop fastener; Velcro; chemistry; theory; chemical bond; Gilbert N. Lewis (1875-1946); valence bond theory; Linus Pauling (1901-1994); resonance (chemistry); resonant bonding; orbital hybridization; engraving; ancient history; seafarers; fishermen; inkscape; Wikimedia Commons; Wellcome Trust; crystals; amorphous solid; noncrystalline; material; freezing; randomness; random; liquid; quenching; rapid cooling; splat cooling; splat quenching; melt spinning; melting; molten; metal; ribbon; metallic glass; amorphous metal; rotation; rotating; heat sink; heat-sinking; flywheel; halite (rock salt); crystallization; concentration; concentrated; brine; salt solution; room temperature; crystal structure; sodium chloride (NaCl); chlorine; anion; Benjah-bmm27; polycrystal; polycrystalline; cooling; rate (mathematics); crystallite; grain; annealing (metallurgy); diffusion; migrate; simulated annealing; optimization (mathematics); silicon; crystal growth; Czochralski method; millimeter; minute; Nd:YAG laser; neodymium-doped YAG; solid-state laser; laser material; hour; chemical element; lattice position; unit cell; nanometer; calculation; calculate; second; velocity; mean thermal velocity; yttrium; Boltzmann constant; Joule; kelvin"; International System of Units; SI base unit; thermodynamic temperature; absolute temperature; mass; melting point; kilogram; kilometer; crystallization; interface (chemistry); scientist; scientific literature; publish; nucleation; Hanyang University (Ansan, Republic of Korea); Korea Advanced Institute of Science and Technology (KAIST, Daejeon, Republic of Korea); Lawrence Berkeley National Laboratory (LBNL, Berkeley, California); Integrated Dynamic Electron Solutions, Inc. (Pleasanton, California); Seoul National University (Seoul, Republic of Korea); Institute for Basic Science (IBS, Seoul, Republic of Korea); Yonsei University (Seoul, Republic of Korea); University of California (Berkeley, California); Kavli Energy NanoSciences Institute (Berkeley, California); dynamics (mechanics); dynamic; reversible process (thermodynamics); reversible; statistical fluctuation; fluctuation; nucleation; nuclei; gold; graphene; substrate; classical physics; energy; Lego®; lifetime; atom cluster; binding energy; exothermic reaction; exothermic process; computer simulation; nanocrystal; redox; reduction; precursor (chemistry); cathode ray; electron beam; electron microscope; frame rate; frames per second; author; Peter Ercius; motion blur; funding of science; funded; National Research Foundation of Korea; United States Department of Energy; Institute for Basic Science (Korea); Samsung Science and Technology Foundation; United States National Science Foundation.