Edison Battery
May 3, 2021
Electric batteries have been used for more than two
centuries. The first battery, called the
voltaic pile, was
invented by
Alessandro Volta in 1800, and our
voltage unit, the
volt, is named in his honor. The voltaic pile was a stack of alternating
copper and
zinc disks separated by
blotting paper soaked in
brine. Brine is a highly
concentrated salt water solution that acts as an
electrolyte.
The voltaic pile is a
primary battery; that is, it is not
rechargeable. The zinc
anode is converted into
doubly-charged zinc
cations (Zn
2+) with two
electrons injected into the zinc
electrode.
Zn -> Zn2+ + 2e-
The zinc cations are balanced by the production of
hydrogen at the copper
cathode. Electrons obtained from the closed
electric circuit combine with the hydrogen cations to form hydrogen gas.
2H+ + 2e- -> H2
The hydrogen gas
bubbles into the
air at the copper cathode. The copper electrode is not
chemically involved in the
electrochemical reaction, which is
Zn + 2H+ -> Zn2+ + H2
The voltaic pile was Volta's most important, but not his only, discovery. He isolated
methane in 1778; and, after the invention of the voltaic pile, he was able to
ignite a methane-air mixture with an
electric spark.
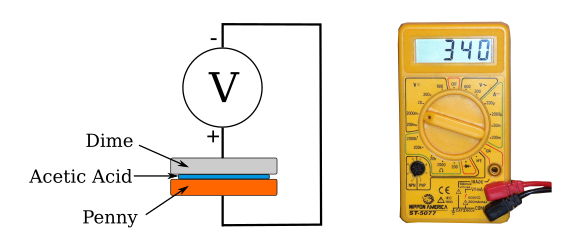
An electrochemical cell, one element of a voltaic pile, with a penny anode, a dime cathode, and a vinegar (acetic acid, CH3CO2H) electrolyte. When I conducted this experiment (millivolt reading on the right), I thought that a US dime was still mostly silver. This shows my age, since the composition of a dime changed from (90% silver, 10% copper), to (91.67% copper, 8.33% nickel) in 1965. A US penny is copper-plated zinc.
My experiment revealed some important hints if you want to build a voltaic pile from coins. First, coins have a ridge around their circumference that requires the blotter paper to be cut to a smaller size. Second, the blotter paper should be thick enough that the coins don't touch under applied pressure. I used a few layers of paper towel material.
(Diagram created using Inkscape.)
Despite our
present aversion to lead, the
rechargeable battery workhorse since the early
20th century has been the
lead-acid battery, invented in 1859. Although this battery type has low
energy-to-
volume and energy-to-
weight ratios, it has qualities, such as low
cost and long
lifetime, that make them suitable for many applications that include
automotive batteries in
non-electric vehicles. It's based on the reaction of lead with
sulfuric acid; viz,
Pb(s) + PbO2(s) + 2H2SO4(aq) -> 2PbSO4(s) + 2H2O(l)
The cell voltage is about 2.05 volts, so six cells are
series connected in an automobile battery to give the
putative 12 volt battery voltage. A typical automobile battery has a capacity of a little more than a hundred
amp-hours. For comparison, a standard
zinc-carbon D-size battery has a capacity of 8 amp-hours.
A
nickel–iron battery was
commercialized by
Thomas Edison more than a century ago. In true Edison style, this battery wasn't invented by Edison. That honor goes to
Swedish inventor,
Waldemar Jungner, but Edison made
money by selling it. He created the
Edison Storage Battery Company in
East Orange, New Jersey that operated from 1903 to 1975. Edison promoted this battery as a
power source for
electric vehicles and
home appliances. I wrote about the Edison battery in an
earlier article (Edison's Nickel-Iron Battery Modernized, July 9, 2012).
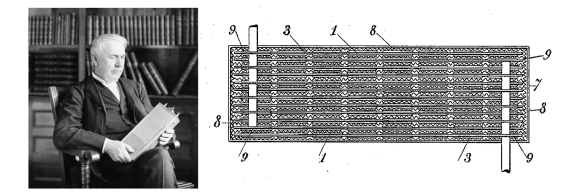
Thomas Edison with his nickel iron battery in 1910, and fig. 4 of US patent No. 692,507, "Reversible Galvanic Battery," by Thomas Alva Edison, February 4, 1902. (Left image, via Wikimedia Commons. Right image, via Google Patents.[1] Click for larger image.)
The nickel–iron battery has a
nickel oxide-hydroxide cathode and an
iron anode. The electrolyte is
potassium hydroxide, often with an addition of
lithium hydroxide. The electrolyte acts merely as a reservoir of mobile
hydroxide ions, and it is not part of the
reaction.
Cathode Reaction:
2NiOOH + 2H2O + 2e− <--> 2Ni(OH)2 + 2OH−
Anode Reaction:
Fe + 2OH− <--> Fe(OH)2 + 2e−
Edison's nickel-iron batteries had a somewhat higher
energy density than lead-acid batteries (up to 50
Wh/kg vs 35-40 Wh/kg).[2] Nickel-iron batteries can be charged twice as fast as lead-acid batteries, and they can endure many
charge/discharge cycles.[2] However, nickel has become an expensive material, selling for about $16/kg as compared with lead's price of about $2/kg.
The
open-circuit voltage of a nickel-iron battery is 1.4 volts, dropping to 1.2 volts during discharge.[2] These cells are typically charged at 1.65 volts, but they have the property that once they're fully charged, continued application of voltage causes the cells to perform
water electrolysis; that is, the electrolyte water is split into hydrogen and
oxygen. This is a problem for batteries; but, as the
management slogan goes, there are no problems, just
opportunities. A
research team from the
Technische Universiteit Delft (Delft University of Technology, Delft, The Netherlands) has turned this effect into a method for production of hydrogen as a
carbon-free fuel.[3-4]
The Delft researchers call their device a
battolyser, a combination of the words, battery and electrolyser, and it solves the problem of intermittent production of energy by
renewable energy sources such as
wind and
solar.[3-4] Conventional batteries can store such intermittent energy; but. when they're fully charged, additional available energy is lost. When the nickel-iron battolyser is fully charged, it can be used to make hydrogen fuel, instead.[4] When the instantaneous electric price is high, the battolyser can feed power into the
electrical grid, and when it's low, the same device can make hydrogen.[4]

Solar photovoltaic and wind power. Left, a 19 megawatt peak photovoltaic system near Thüngen, Bavaria, Germany. Right, the 40 megawatt Middelgrunden offshore wind farm at Øresund strait near Copenhagen, Denmark. (Left image by Oh Weh, and right image by Kim Hansen, both from Wikimedia Commons. Click for larger image.)
In operation as an electrolyser,
nanostructured NiO(OH) and Fe coat the electrode surfaces, and these act as good
catalysts for oxygen and hydrogen production.[3] The battolyser electrodes are
robust in electrolysis, while electrodes in traditional batteries are degraded, and their
energy conversion efficiency is in the range of 80-90%.[4] Aside from hydrogen, such cells can be designed to generate
ammonia or
methanol, two fuels that are easier to store than hydrogen.[4]
At this time, the researchers have produced a 15 kW/15 kWh battolyser which has storage potential for powering one and a half
households. They are making a 30 kW/30 kWh battolyser for the Magnum power station in
Eemshaven, Netherlands.[4] Scaling to
gigawatts would solve the storage needs of a
wind farm, but smaller units can serve
rural areas away from the main power grids.[4] Areas for further research are reduction in the cell
internal resistance to improve efficiency.[4]
References:
- Thomas Alva Edison, "Reversible Galvanic Battery," US Patent No. 692,507, February 4, 1902 (via Google Patents).
- Nickel-Iron Battery Website.
- F. M. Mulder, B. M. H. Weninger, J. Middelkoop, F. G. B. Ooms, and H. Schreuders, "Efficient electricity storage with a battolyser, an integrated Ni–Fe battery and electrolyser," Energy & Environmental Science. vol. 10, no. 3 (December 14, 2016), pp. 756-764, https://doi.org/10.1039/C6EE02923J.
- Allison Hirschlag, "The battery invented 120 years before its time," BBC, February 24, 2021.
- Battolyser Web Site.
Linked Keywords: Electric battery; century; voltaic pile; invention; invented; Alessandro Volta; voltage unit; volt; copper; zinc; disk (mathematics); blotting paper; brine; concentration; concentrated; salt water; aqueous solution; electrolyte; primary cell; primary battery; rechargeable battery; anode; electric charge; doubly-charged; cation; electron; electrode; hydrogen; electrical network; electric circuit; bubble (physics); atmosphere of Earth; air; chemical reaction; chemical; electrochemistry; electrochemical; methane; combustion; ignite; electric spark; electrochemical cell; penny (United States coin); dime (United States coin); vinegar; acetic acid; CH3CO2H; experiment; millivolt; silver; baby boomer; alloy; composition; nickel; plating; plated; coin; circumference; pressure; paper towel; material; Inkscape; lead poisoning; present aversion to lead; workhorse; 20th century; lead-acid battery; energy; volume; mass; weight; ratio; cost; service life; lifetime; automotive battery; internal combustion engine; non-electric vehicle; series circuits; series connected; putative; ampere hour; amp-hour; zinc-carbon battery; D-size battery; nickel–iron battery; commercialization; commercialize; Thomas Edison; Swedish; inventor; Waldemar Jungner; money; Edison Storage Battery Company; East Orange, New Jersey; electric power; electric car; electric vehicle; home appliance; US patent; Wikimedia Commons; Google Patents; nickel oxide-hydroxide; iron; potassium hydroxide; lithium hydroxide; hydroxide ion; energy density; watt-hour per kilogram; Wh/kg; charge/discharge cycles; open-circuit voltage; water electrolysis; oxygen; management; slogan; market opportunity; research; Technische Universiteit Delft (Delft University of Technology, Delft, The Netherlands); carbon-neutral fuel; carbon-free fuel; renewable energy sources; wind power; solar power; electrical grid; photovoltaic system; solar photovoltaic; megawatt; Thüngen; Bavaria, Germany; Middelgrunden offshore wind farm; Øresund; strait; Copenhagen, Denmark; Oh Weh; Kim Hansen; nanostructure; NiO(OH); catalysis; catalyst; resilience; robust; energy conversion efficiency; ammonia; methanol; households; Eemshaven, Netherlands; gigawatt; wind farm; rural area; internal resistance; Thomas Alva Edison, "Reversible Galvanic Battery," US Patent No. 692,507, February 4, 1902.