Thermoelectrics
October 26, 2020
As the
First law of thermodynamics informs us, a
heat engine will only provide
work through a difference in
temperature between two
thermal reservoirs. A heat engine is not the only way to extract work from a temperature differential. Work can be extracted from a temperature differential in the form of
electrical power by using the
thermoelectric effect; specifically, the
Seebeck effect.
The simplest thermoelectric device based on the Seebeck effect is the
thermocouple, which is just a junction between two dissimilar
metals. Some metal pairs offer a greater
voltage change per
degree than others. A popular thermocouple is the
Type K, which is a junction between
chromel, an
alloy that's 90%
nickel and 10%
chromium and
alumel, an alloy of 95% nickel, 2%
aluminum, 2%
manganese, and 1%
silicon. This thermocouple has a
thermopower (sensitivity) of about 41 μV/°C.
A thermopower of 41 μV/°C, combined with
electronic amplification, makes thermocouples useful for temperature measurement. Additionally, electrical power can be extracted from them with a little effort. This would be done by stringing many of these in
series, so their voltages add. There's one caveat, however. Each
heated thermocouple junction must be matched by a
cold junction, as required by the first law of thermodynamics (see figure).
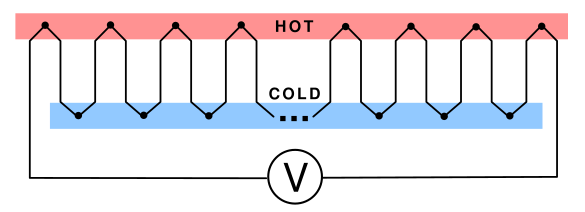
A series connection of many thermocouple junctions intended as a voltage source. Useful voltages will be generated only with a large temperature difference between the hot and cold junctions and many thermocouples are used. Since the power output of such a device depends on its internal resistance as well as the voltage, there are practical problems, as explained in the text. (Created using Inkscape.)
One of my
sixth grade classmates made an
electrical generator this way by putting the cold junctions in an
ice bath, and heating the hot junctions with a
propane torch. The intended use of such a device was as a
solar energy system in which the hot junctions would be at the
focus of a
parabolic mirror. A hundred junctions with a several hundred degree temperature difference will generate enough voltage to barely light an
incandescent flashlight bulb.
The problem with this thermoelectric approach is that metals that are good electrical conductors are also good
thermal conductors, as explained by the
Wiedemann–Franz law. That's a consequence of
electrons' being responsible for both conductivies. Fortunately,
specially crafted semiconductors can be used to make somewhat useful
thermoelectric generators.
Junctions of
n- and
p-doped bismuth telluride (Bi2Te3) are typically used in thermoelectric cells, as shown in the figure.
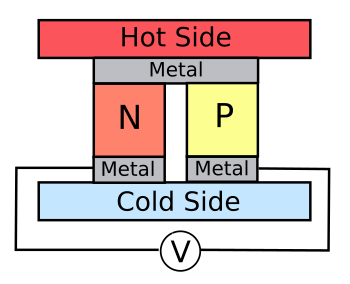
A thermoelectric cell of n- and p-doped semiconductor.
A series connection of many such cells offer a means to extract useful electrical power from sources of waste heat, such as automobile exhaust gas.
(Click for larger image.)
Bismuth telluride thermoelectric generators are relatively
inefficient, so there are efforts to find better
materials. Obviously, good thermoelectric performance, as identified by a high
Seebeck coefficient (
S, volts/kelvin), and low
thermal conductivity (κ,W/(m-K)) are both beneficial. These are combined into a
thermoelectric figure of merit, commonly denoted as
zT, as follows:
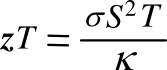
In this equation,
σ is the
electrical conductivity. Somewhat confusingly,
zT, is used as the figure of merit, with temperature multiplied to get a dimensionless number. That's why we don't just cancel the temperature term from both sides of the
equation. The figure of merit changes slightly with temperature, and the highest figures of merit have been just slightly above 1.0 with no great advances over the years. However, a figure of merit of 1.0 is far greater than my
calculated value for a type-K thermocouple, 0.05, obtained by using
averaged values of the
properties of chromel and alumel.
An advance in thermoelectric figure of merit has been achieved by
scientists and
engineers from the
Huazhong University of Science and Technology (Wuhan, China),
Wuhan University (Wuhan, China), and the
Chinese Academy of Sciences (Beijing, China).[1-2] Their device, a
liquid-state thermocell, is a variation on an effect most recently studied by
Japanese scientists.[3] The Japanese
researchers describe what they call a
tertiary battery, a new
battery type additional to
primary (single use) batteries and
secondary (rechargeable) batteries. These tertiary batteries exhibit a temperature related voltage
hysteresis that can be used for thermal
energy harvesting (see graph).
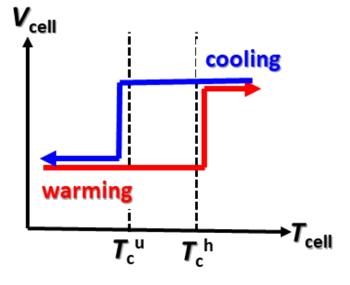
Temperature related voltage hysteresis in a "tertiary battery."
This effect can be used for thermal energy harvesting.
Temperature cycling must be between a lower critical temperature (Tcl) and an upper critical temperature (Tcu).
(fig. 4(d) of ref. 3, licensed under a Creative Commons Attribution 4.0 International License.[3])
The Japanese tertiary battery is enabled by a
phase transition at one of the
electrodes.[3] The transition occurs in the
cobalt Prussian blue analogue, Na
xCo[Fe(CN)
6]
y, the
composition of which is tuned so that the transition happens around
room temperature.[3] The entire cell is thermally cycled in that device, while the liquid-state thermocell operates as a usual thermoelectric cell in contact with cold and hot thermal reservoirs.
Unlike
solid state thermoelectric cells, like those made from bismuth telluride, the liquid-state thermocell transports
electrical charge by means of
ions instead of electrons, and such ions are less effective at charge transport than electrons. Ions likewise still conduct heat, so the figure of merit and
energy conversion efficiency are low.[2] The liquid-state thermocell overcomes these problems by using temperature sensitive
crystallization and
dissolution of a material.[1-2] In this fashion, a Seebeck coefficient of 3.73 mV/K was achieved with low thermal conductivity.[1]
The cell liquid is a
ferricyanide (
Fe(
CN)
6)
solution that transfers electrons to the hot electrode, and receives electrons at the cold electrode by the following reactions:
Hot: Fe(CN)6-4 -> Fe(CN)6-3 + e-
Cold: Fe(CN)6-3 + e- -> Fe(CN)6-4
The electric current is conducted through an
external circuit to provide power (see figure).[2]
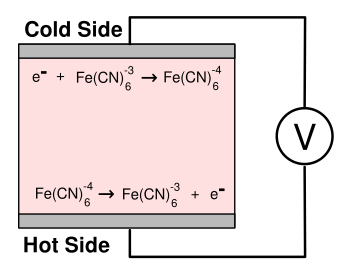
A liquid-state thermocell based on ferricyanide ions.
(Created using Inkscape. Click for larger image.)
The thermal conductivity of the liquid is reduced by adding the positively charged
organic compound,
guanidinium.[2] This causes the ferricyanide to crystallize at the cold electrode, thereby thermally insulating it.[2] The crystallites are
precipitated by
gravity down to the hot electrode, which is hot enough to liquify them.[2]
Experiments using a 50°C temperature differential gave a
Carnot efficiency of about 11%, which is more than double the efficiency needed for a viable
commercial device.[1-2] Stringing twenty of these cells in series provided sufficient voltage and power to
charge a
mobile phone.[1-2]
References:
- Boyang Yu, Jiangjiang Duan, Hengjiang Cong, Wenke Xie, Rong Liu, Xinyan Zhuang, Hui Wang, Bei Qi, Ming Xu, Zhong Lin Wang, and Jun Zhou, "Thermosensitive crystallization–boosted liquid thermocells for low-grade heat harvesting," Science, Article no. eabd6749, September 10, 2020, DOI: 10.1126/science.abd6749.
- Robert F. Service, "Energy 'scavenger' could turn waste heat from fridges and other devices into electricity," Science, September 11, 2020, doi:10.1126/science.abe7571.
- Takayuki Shibata, Hiroki Iwaizumi, Yuya Fukuzumi & Yutaka Moritomo, "Energy harvesting thermocell with use of phase transition," Scientific Reports, vol. 10 (February 4, 2020), Article no. 1813, https://doi.org/10.1038/s41598-020-58695-z. This is an open access paper with a PDF file here
Linked Keywords: First law of thermodynamics; heat engine; work (physics); temperature; thermal reservoir; electrical power; thermoelectric effect; Seebeck effect; thermocouple; metal; voltage; Celsius; degree; chromel; alloy; nickel; chromium; alumel; aluminum; manganese; silicon; Seebeck coefficient; thermopower; electronic amplification; amplifier; series circuit; heat; heated; series connection; thermocouple junction; voltage; electric power; resistance; Inkscape; sixth grade; classmate; electrical generator; cooling bath; ice bath; propane torch; solar energy; focus (optics); paraboloid; parabolic; mirror; incandescent flashlight bulb; thermal conductivity; thermal conductor; Wiedemann–Franz law; electron; thermoelectric generator materials; semiconductor; thermoelectric generator; n-type semiconductor; p-type semiconductor; bismuth telluride (Bi2Te3); thermoelectric cell; waste heat; automobile; exhaust gas; energy conversion efficiency; inefficient; material; thermoelectric figure of merit; electrical conductivity; equation; calculation; calculated; average; materials properties; scientist; engineer; Huazhong University of Science and Technology (Wuhan, China); Wuhan University (Wuhan, China); Chinese Academy of Sciences (Beijing, China); Japan; Japanese; research; researcher; battery (electricity); primary cell; primary (single use) battery; rechargeable battery; secondary (rechargeable) battery; hysteresis; energy harvesting; temperature; voltage; Creative Commons Attribution 4.0 International License; phase transition; electrode; cobalt; Prussian blue; analogy; analogue; chemical composition; room temperature; solid state; electrical charge; ion; crystallization; dissolution (chemistry); ferricyanide; iron; Fe; cyanide; CN; aqueous solution; external circuit; Inkscape; organic compound; guanidinium; precipitation (chemistry); precipitate; gravitational field; gravity; experiment; Carnot's theorem (thermodynamics); Carnot efficiency; commerce; commercial; battery charger; charge; mobile phone.