Neutron Dipole Moment
April 27, 2020
The
concept of
matter was simple in
Aristotle's time, around 350
BC, there being just
four elements;
Earth (Γη),
Air (ʾΑηρ),
Fire (Πυρ), and
Water (ʿΥδωρ). All things were
composites of different
proportions of these four fundamental elements. The problem with Aristotle's approach, however, is that none of this was determined by
experiment, just
pure thought.
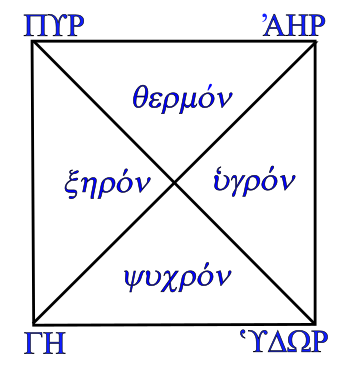
There were four qualities, Dry ξηρον, Wet ʿυγρον, Hot θερμον, and Cold ψυχρον associated with the four classical elements, Earth ΓΗ, Air ʾΑΗΡ, Fire ΠΥΡ, and Water ʿΥΔΩΡ.
Fire is both hot and dry, Earth is both cold and dry, Air is both hot and wet, and water is both cold and wet.
Composite materials built from these pure elements had qualities in proportion to their Earth-Air-Fire-Water content.
(Created using Inkscape. Click for larger image.)
A contrary view, that
matter is composed of indivisible particles called
atoms, was popular even before Aristotle's time; but, that
theory (
atomism) was also just a product of pure thought. Much later, a multitude of actual
chemical elements was discovered using scientific methods, starting with the discovery of
phosphorous by
German Alchemist,
Hennig Brand in 1649. In the
19th century,
English chemist,
John Dalton, gave
scientific evidence that atoms do exist, and that
chemical reactions involve the
separation or rearrangement of atoms.
Eventually,
physicists began to wonder whether the atoms of
chemical elements were composites of other things. In 1897, the first constituent of atoms, the
electron was discovered by
Joseph John Thomson (1856-1940, commonly known as "J.J. Thomson"), for which Thomson was awarded the 1906
Nobel Prize for Physics. Thomson's model of matter was the so-called
plum-pudding model in which the electrons were embedded like
raisins in a
positively-
charged pudding. The electron
raisins were allowed to
orbit within the positive
pudding.
Interestingly, while most scientists in the later part of the
19th century conjectured that any constituent parts of
atoms should also be of
atomic size, Thomson's
experiments showed that the
negatively-
charged cathode rays he had discovered were a thousand times smaller. This result indicated a need for a deeper probing of the atom.
Experiments by
Ernest Rutherford in which he
analyzed the
scattering of
alpha particles striking
gold foils revealed the existence of a compact positively-charged
nucleus. In 1911, Rutherford proposed a
planetary model of the atom, refined by
Niels Bohr (1885-1962), whose
model explained the
emission spectra of atoms. In experiments from 1917-1925, Rutherford proved that the atomic nucleus contains the nuclei of
hydrogen atoms, which marks the discovery of the
proton.

Ernest Rutherford (1871-1937).
While Rutherford is famous for the discovery of the proton, his 1908 Nobel Prize was in chemistry "For his investigations into the disintegration of the elements, and the chemistry of radioactive substances."
Rutherford was Director of the Cavendish Laboratory of Cambridge University when James Chadwick discovered the neutron in 1932.
(Wikimedia Commons image, modified for artistic effect.)
Experimental science advances as improved
laboratory equipment becomes available. It took
James Chadwick (1891-1974) just two weeks of experiments with
neutrons to feel confident enough to send a
letter to
Nature in February, 1932, entitled, "Possible Existence of a Neutron". He followed the letter with a detailed
article in the
Proceedings of the Royal Society A entitled, "The Existence of a Neutron." Chadwick was quickly awarded the 1935
Nobel Prize in Physics for this discovery.
At the time of its discovery, there was the idea that neutrons might be composite particles consisting of an electron
bound to a proton, the equal charges balancing to zero. Chadwick and his
colleague,
Maurice Goldhaber (1911-2011), found that the
neutron mass of 1.0084-1.0090
daltons (now known to be 1.008645 daltons) was too large for that possibility; so, at that time, electrons, protons, and neutrons were considered to be
elementary particles and not composite particles.
As science advanced into the
1960s and
1970s, there was a realization that protons and neutrons might be composite particles, In 1968,
accelerator experiments indicated that protons might contain smaller objects. Eventually, physicists developed what's called the
Standard Model that has protons and neutrons constructed from three tightly-bound particles called
quarks (see figure). Everything needs a name, and
the quark was named by physicist,
Murray Gell-Mann (1929-2019), who took the word from a
phrase in
Finnegans Wake by
James Joyce (
Three quarks for Muster Mark!, which is commonly considered to mean,
Three quarts for Mister Mark!, the quart being a
measure of
beer. Since three quarks make a proton, the appearance of "three" in that phrase probably helped.)
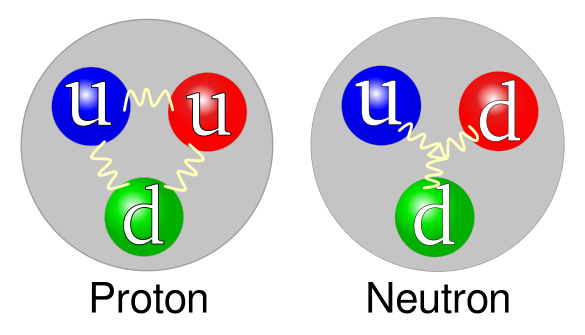
Quark model for the proton and neutron. The two types of quarks present in each are the "up" quark u and the "down" quark d. (Left, Wikimedia Commons image by Arpad Horvath. Right, Wikimedia Commons image by Jacek Rybak.)
Even with its quark structure, the neutron is firmly without charge; but, just like the early electron-proton model of the neutron in which internal charges balance to zero, could that also be the case for the neutron? When looking for something like that, physicists are looking for an
electric dipole moment of the neutron, which reflects the
distribution of positive and negative charge inside the neutron. A zero dipole moment indicates that any positive and negative charges internal to the neutron coincide.
A neutron electric dipole moment above a very small limit would reveal problems with our present understanding of
particle physics. The experimental method for measuring the moment is facilitated by the neutron's having a
magnetic moment that
precesses at an easily detected
frequency called the
Larmor frequency. I did an
undergraduate project in
electron paramagnetic resonance that utilized this same principle. In the case of electrons, the frequency varies with
magnetic field as 28,025 MHz/T, and
microwave frequencies are employed with conventional
electromagnets. For the neutron, the frequencies are the much smaller 42.576 MHz/T, so the larger magnetic fields from
superconducting magnets are typically employed.
The dipole momemnt experiment involves measuring this frequency for neutrons in the presence of a magnetic field while flipping the direction of an applied
electric field. If there's no dipole moment, there is no change in frequency. An accurate measurement is only obtained when the magnetic field is stable and the
motion of neutrons in the measured
neutron beam is small. The first such measurement was done in 1957 by a team of physicists that included
Edward M. Purcell (1912-1997), who had shared the 1952 Nobel Prize for Physics for his 1946 discovery of
nuclear magnetic resonance, and
Norman F. Ramsey (1915-2011), who was subsequently awarded the 1989 Nobel Prize in Physics for work on
atomic clocks.[1] This first measurement indicated that the neutron electric dipole moment was less than 5x10
-20 e-cm, and it was the first of many such measurements of improved accuracy (see graph).[2]
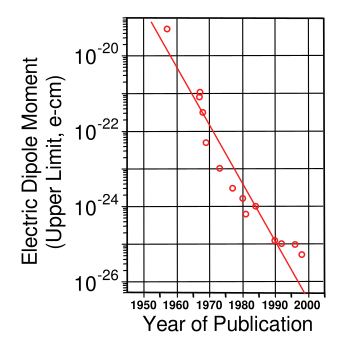
Experimental upper limit of neutron electric dipole moment vs year.
The first data point, at the top left of this graph, is the Smith-Purcell-Ramsey experiment of 1957. Sensitivity has increased about six orders of magnitude since that time.
(Created using Inkscape from data on page 10 of ref. 2.[2])
The most recent measurement of the neutron electric dipole moment was conducted by a huge international team of 84 scientists from 18
research institutions in
Belgium,
France,
Germany,
Poland,
Serbia,
Switzerland, the
United Kingdom, and the
United States.[3-4] Their result, that the moment is less than 1.8x10
-26 e-cm, is
published in an
free, open access paper in
Physical Review Letters.[3-4] This is about half the best previous value, and it was obtained by increasing the time that neutrons could be observed.[4] Control of the magnetic field needed to achieve this accuracy was facilitated by
atomic magnetometers based on the
199Hg isotope and
cesium vapor.[3] The research team for this experiment was led by
Philipp Schmidt-Wellenburg of the
Paul Scherrer Institute and
Guillaume Pignol of the
Laboratoire de Physique Subatomique et de Cosmologie in France.[4]
So, why is this important? The Standard Model is not complete, since it doesn't combine
quantum mechanics and
general relativity, and there's the further problem of why the
universe has more matter than
antimatter. It's thought that a more complete theory would show that
charge-parity (CP) symmetry is not conserved in quark binding by the
strong force.[4] Such
CP violation in the
energetic, early universe would give more matter than antimatter, and this possibility would be exposed in a neutron electric dipole moment.[4] The observed limit of the moment indicates that CP violation of the strong force may have occurred earlier than the time when
electromagnetic and weak nuclear forces became distinct.[4]
References:
- J. H. Smith, E. M. Purcell, and N. F. Ramsey, "Experimental Limit to the Electric Dipole Moment of the Neutron," Phys. Rev., vol. 108, no. 1 (October, 1957), pp. 120ff., DOI:https://doi.org/10.1103/PhysRev.108.120.
- J. Pretz, "Measurement of Permanent Electric Dipole Moments of Proton, Deuteron and Light Nuclei in Storage Rings," Forschungszentrum Jülich Website, June 18, 2012 (PDF File).
- C. Abel et al., "Measurement of the Permanent Electric Dipole Moment of the Neutron," Phys. Rev. Lett., vol. 124, no. 8 (February 28, 2020), Article no. 081803, https://doi.org/10.1103/PhysRevLett.124.081803. This is an open access paper with a PDF file here.
- Philip Ball, "Focus: New Limit on the Neutron’s Internal Charge Asymmetry," Physics, vol. 13, no. 25 (February 28, 2020).
Linked Keywords: Concept; matter; Aristotle; Anno Domini; BC; classical element; four elements; Earth (classical element); Air (classical element); Fire (classical element); Water (classical element); composite material; ratio; proportion; experiment; thought experiment; pure thought; four qualities of matter; quality (philosophy); arid; dry; humidity; wet; heat; hot; cold; Inkscape; indivisible particle; atom; theory; atomism; timeline of chemical element discoveries; phosphorous; German; Alchemy; Alchemist; Hennig Brand; 19th century; English; chemist; John Dalton; science; scientific; chemical reaction; separation process; physicist; chemical elements; electron; J. J. Thomson; Joseph John Thomson; Nobel Prize for Physics; plum-pudding model; raisin; electrical polarity; positive; electric charge; charged; pudding; atomic orbital; orbit; 19th century; conjecture; atom; atomic radius; atomic size; negative; cathode ray; analysis; analyze; Rutherford scattering; alpha particle; gold; foil (metal); atomic nucleus; Rutherford model; planetary model of the atom; Niels Bohr (1885-1962); Bohr model; emission spectra; hydrogen; proton; Ernest Rutherford (1871-1937); Nobel Prize was in chemistry; radioactive decay; disintegration; chemistry; radioactive; Director; Cavendish Laboratory; University of Cambridge; Cambridge University; laboratory equipment; James Chadwick (1891-1974); letter (message); Nature (journal); scientific literature; article; Proceedings of the Royal Society A; binding energy; bound; colleague; Maurice Goldhaber (1911-2011); neutron mass; dalton (unit); elementary particle; 1960s; 1970s; particle accelerator; Standard Model; quark; quark etymology; Murray Gell-Mann (1929-2019); phrase; Finnegans Wake; James Joyce; quart; volume; measure; beer; quark model; quark; "up" quark; "down" quark; Wikimedia Commons; Jacek Rybak; electric dipole moment of the neutron; charge density; distribution; particle physics; magnetic moment; precession; precess; frequency; Larmor frequency; undergraduate education; electron paramagnetic resonance; magnetic field; microwave; electromagnet; superconducting magnet; electric field; motion (physics); particle beam; neutron beam; Edward M. Purcell (1912-1997); nuclear magnetic resonance; Norman F. Ramsey (1915-2011); atomic clock; neutron electric dipole moment; data; Cartesian coordinate system; graph; orders of magnitude; Inkscape; research institute; Belgium; France; Germany; Poland; Serbia; Switzerland; United Kingdom; United States; scientific literature; publish; open-access journal; free, open access paper; Physical Review Letters; SERF; atomic magnetometer; isotope of mercury; 199Hg; cesium; vapor; Philipp Schmidt-Wellenburg; Paul Scherrer Institute; Guillaume Pignol; Laboratoire de Physique Subatomique et de Cosmologie; quantum mechanics; general relativity; universe; antimatter; CP violation; charge-parity (CP) symmetry; strong interaction; strong force; energy; energetic; J. H. Smith, E. M. Purcell, and N. F. Ramsey, "Experimental Limit to the Electric Dipole Moment of the Neutron," Phys. Rev., vol. 108, no. 1 (October, 1957), pp. 120ff., DOI:https://doi.org/10.1103/PhysRev.108.120.