Printed Piezoelectrics
October 29, 2018
Microphones are an integral part of many of today's
electronic devices. One obvious locale for a microphone is your
cellphone, but there are now microphones in
consumer electronic devices such as
Google Home, the
Amazon Echo, and the remote control of
Amazon Fire TV that I use to watch far too many
science fiction films of the
1950s. I enjoy these films, since these were the films of my
childhood.
The microphone type in all these devices is the
electret microphone, a type that's easily miniaturized and fabricated using
planar processing technology. My first microphone, which was an accessory for an inexpensive
tape recorder, was a
crystal microphone. The active element of this microphone was the
piezoelectric material,
potassium sodium tartrate tetrahydrate, commonly called Rochelle salt after the place of its first synthesis in about 1675. Just like a
crystal wine goblet, the crystal of this microphone was destroyed when the microphone was dropped onto the
floor.
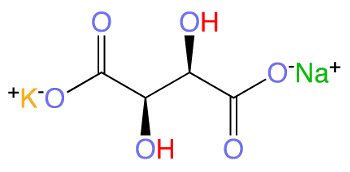
Structural diagram of L-Rochelle salt, potassium sodium tartrate tetrahydrate, KNaC4H4O6.
This inorganic salt typically exists with four waters of hydration.
(Modified Wikimedia Commons image.)
Piezoelectrics are useful for more than just microphones. They also perform the inverse function of turning an
oscillating electric field into
mechanical movement. In its simplest form, this would be a
loudspeaker, but a piezoelectric can be used as an
actuator or
piezoelectric motor. Unfortunately, the mechanical
force is relatively small, primarily because the
high impedance of a piezoelectric doesn't allow much of a
power transfer at reasonable
voltages. Piezoelectrics can also be used as
vibration energy harvesters, converting
environmental vibration and things such as the force exerted by a gentle
breeze to
electric power for miniature devices.
Piezoelectrics are also used as
resonant elements in
frequency sources, and
quartz, a piezoelectric
mineral available as large
crystals in
nature and later as
laboratory grown crystals, has been used in this application since the advent of
radio. While thin
wafers of quartz were the predominant piezoelectric resonator for a
century,
surface acoustic wave resonators of other piezoelectric materials such as
lithium niobate have become popular now that radio technology has extended to higher frequencies, such as 5.8
gigahertz WiFi.

Quartz crystal in a dolomite ore, a "Herkimer diamond" from Herkimer County, New York.
Such quartz crystals were formed in pockets of acidic aqueous solutions of silica in the Carboniferous Period.
This particular crystal growth mechanism, which is used to grow quartz and other crystals in a laboratory, is called hydrothermal synthesis.
(Wikimedia Commons image by Robert Lavinsky.)
Materials scientists are always searching for
materials having better properties than existing materials. One property that's important for a piezoelectric material is its
electromechanical coupling coefficient, the quantity that relates how well the material can convert
electrical energy into
mechanical energy, and it varies widely between materials and their
crystallographic directions. There are piezoelectrics other than quartz that are used in their crystalline form, many with properties that are superior to quartz. Among these are
lithium tantalate (LiTaO3),
lithium niobate (LiNbO3),
barium titanate (BaTiO3),
lithium tetraborate (Li2B4O7),
aluminum phosphate (aluminum phosphate (AlPO4,
Berlinite),
gallium phosphate (GaPO4),
lead magnesium niobate (PMN), and
lead zirconate titanate (PZT).
While slices of single crystals offer the best piezoelectric conversion of electrical power to mechanical motion, and vice-versa,
textured ceramic layers of piezoelectric materials will give a response. They're only about a tenth as
efficient, but that's often enough in some applications. Films of PZT,
Zinc oxide (ZnO) or
aluminum nitride (AlN) are sometimes layered onto
semiconductor wafers to give an added piezoelectric functionality. In particular, AlN is used to fabricate
thin-film bulk acoustic resonators for
microwave filters.

As easy as this - An aluminum nitride (AlN) thin-film bulk acoustic resonator (FBAR) on silicon.
The speed of longitudinal acoustic wave in AlN is about 10,000 meters/second, so a resonant half wavelength in a one micrometer layer corresponds to a frequency of about 20 GHz.
(Created using Inkscape.)
The usual deposition process for piezoelectric thin films is
physical vapor deposition, a
vacuum deposition process that has a large enough deposition rate to prepare micrometer thickness layers, as in the
FBAR example in the above figure. A method of
printing piezoelectrics would allow an easier production of large area and thicker layers. That was the object of
research on the piezoelectric, gallium phosphate (GaPO
4) by a team of
Australian and
German scientists from the
Royal Melbourne Institute of Technology (RMIT, Melbourne, Australia), the
University of New South Wales (New South Wales, Australia), and the
University of Münster (Münster, Germany). This research is reported in an
open access paper in
Nature Communications.[1-2]
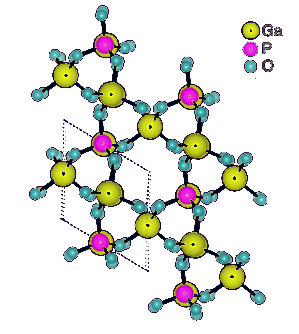
Structure of gallium phosphate (GaPO4) at unit cell thickness.
Gallium phosphate (α)-GaPO4), is a piezoelectric material that is iso-structural with α-quartz.
It has trigonal symmetry with cell parameters of a = 0.487 nm and c = 1.105 nm and trigonal angle γ = 120°.[1]
(Australian Research Council Centre of Excellence in Future Low-Energy Electronics Technologies image, modified.)
The process, as shown in the figure, is quite simple. A substrate is first
wetted with a thin layer of
gallium oxide (Ga2O3) by contact with the normally
oxidized surface of
liquid gallium.
Van der Waals forces cause the
exfoliation and
adsorption of the gallium oxide layer.[2] This step is easy, since the
melting point of gallium is just 29.77
°C. This film of gallium oxide is then exposed to flowing
vapors of
phosphoric acid at 350 °C, a very low
temperature for wafer processing.[1-2]
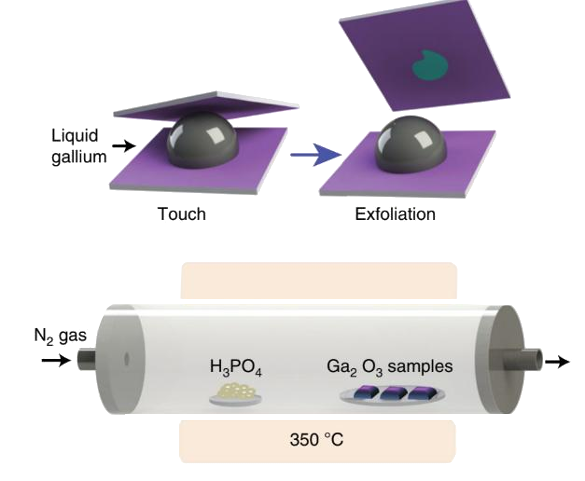
Process for creation of gallium phosphate layers. Van der Waals forces allow transfer of the gallium oxide layer from a liquid droplet of gallium to a substrate. A chemical vapor reaction transforms the gallium oxide to gallium phosphate nanosheets.(Australian Research Council Centre of Excellence in Future Low-Energy Electronics Technologies image.)
Unit cell nanosheets showed a large
out-of-plane piezoelectric coefficient of 7.5 ± 0.8
picometers/
volt.[1-2] Bulk gallium phosphate has a significantly higher thermal stability and a higher piezoelectric coefficient than quartz, so the same might be expected for these nanosheets.[1] The α-phase of bulk gallium phosphate is stable up to 930 °C.[1]
This
synthesis technique produces gallium phosphate nanosheets of unit cell thickness that could potentially reach
centimeter dimension.[1] This printing process is also suitable for creation of free-standing gallium phosphate nanosheets.[1]
computational support for this study was provided by the
Pawsey Supercomputer Centre, named after the Australian
radio astronomer,
Joseph Lade Pawsey (1908-1962).[2]
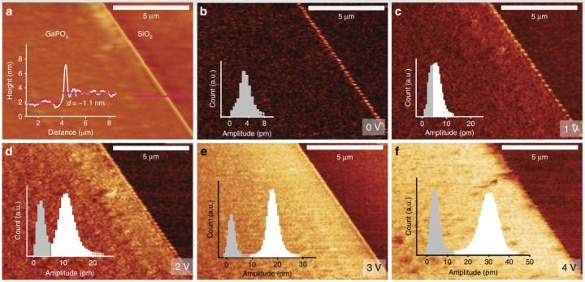
Atomic force microscope imaging of the gallium phosphate nanosheets, including piezoelectric measurements at various applied voltages. (Australian Research Council Centre of Excellence in Future Low-Energy Electronics Technologies image.)
References:
- Nitu Syed, Ali Zavabeti, Jian Zhen Ou, Md Mohiuddin, Naresh Pillai, Benjamin J. Carey, Bao Yue Zhang, Robi S. Datta, Azmira Jannat, Farjana Haque, Kibret A. Messalea, Chenglong Xu, Salvy P. Russo, Chris F. McConville, Torben Daeneke. and Kourosh Kalantar-Zadeh, "Printing two-dimensional gallium phosphate out of liquid metal," Nature Communications, v. 9, Article no. 3618 (2018), DOI 10.1038/s41467-018-06124-1. This is an open access paper with a PDF file here.
- Pushing 'print' on large-scale piezoelectric materials, Australian Research Council Centre of Excellence in Future Low-Energy Electronics Technologies Press Release, September 6, 2018.
Linked Keywords: Microphone; electronic device; mobile phone; cellphone<; consumer electronic device; Google Home; Amazon Echo; Amazon Fire TV; science fiction film; 1950s; childhood; electret microphone; planar processing technology; tape recorder; crystal microphone; piezoelectricity; piezoelectric; potassium sodium tartrate tetrahydrate; lead glass; crystal wine goblet; floor; structural formula; structural diagram; levorotation; L-Rochelle salt, potassium sodium tartrate tetrahydrate; potassium; sodium; carbon; hydrogen; oxygen; inorganic compound; salt; water of crystallization; waters of hydration; Wikimedia Commons; oscillation; oscillating; electric field; mechanics; mechanical; loudspeaker; actuator; piezoelectric motor; force; high impedance; energy transformation; power transfer; voltage; vibration energy harvester; environment; environmental; acoustics; vibration; wind; breeze; electric power; resonance; resonant; frequency source; quartz; mineral; crystal; nature; crystal growth; laboratory grown crystal; radio; wafer (electronics); century; surface acoustic wave; SAW resonator; lithium niobate; gigahertz; WiFi; dolostone; dolomite ore; Herkimer diamond; Herkimer County, New York; acid; acidic; aqueous solution; silicon dioxide; silica; Carboniferous Period; hydrothermal synthesis; Robert Lavinsky; materials science; materials scientist; material; electromechanical coupling coefficient; electrical energy; mechanical energy; Miller index; crystallographic direction; lithium tantalate (LiTaO3); lithium niobate (LiNbO3); barium titanate (BaTiO3); lithium tetraborate (Li2B4O7); aluminum phosphate (aluminum phosphate (AlPO4, Berlinite); gallium phosphate (GaPO4; lead magnesium niobate (PMN); lead zirconate titanate (PZT); texture (crystalline); ceramic; energy conversion efficiency; efficient; Zinc oxide (ZnO); aluminum nitride (AlN); semiconductor; thin-film bulk acoustic resonator; microwave filter; silicon; speed; longitudinal acoustic wave; meters/second; half wavelength; micrometer; frequency; GHz; Inkscape; physical vapor deposition; vacuum; FBAR; printing; research; Australian; German; scientist; Royal Melbourne Institute of Technology (RMIT, Melbourne, Australia); University of New South Wales (New South Wales, Australia); University of Münster (Münster, Germany); open access journal; open access paper; Nature Communications; molecular structure; unit cell; iso-structural; α-quartz; trigonal crystal system; trigonal symmetry; wetting; wetted; oxide; oxidized; surface; liquid; gallium; van der Waals force; exfoliation; adsorption; melting point; Celsius; °C; vapor; phosphoric acid; temperature; gallium oxide; droplet; substrate; chemical vapor deposition; chemical vapor; chemical reaction; nanoscopic scale; nanosheet; Australian Research Council Centre of Excellence in Future Low-Energy Electronics Technologies; normal (geometry); out-of-plane; picometer; volt; chemical synthesis; centimeter; computation; computational; Pawsey Supercomputer Centre; radio astronomy; radio astronomer; Joseph Lade Pawsey (1908-1962); atomic force microscope.