Molecular Shapes
March 26, 2018
Iron atoms are
chemically indistinct, but iron as we see it can be different
materials. That's because these atoms can array themselves into different
crystal structures with different
properties. It's possible to see iron change into three
allotropes just by
heating at
atmospheric pressure (1
bar, see figure). By
convention, allotropes are designated by
Greek letters, with α- (alpha-) being the form at lowest temperature, proceeding up the
alphabet (β-, γ-, δ-, etc,). This has
embarrassed a few
scientists when an even lower temperature form of an
element was discovered.
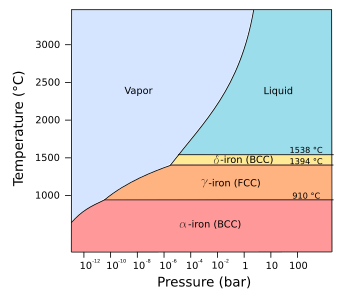
Phase diagram of pure iron.
(Modified Wikimedia Commons image by Daniele Pugliesi. Click for larger image.)
The iron
phase most familiar, since it's the one we can hold in our
hands, is
α-iron, also called ferrite, that has the
body-centered cubic crystal structure. Ferrite exists from the lowest temperatures up to 912
°C, and it's
magnetic up to its
Curie temperature of 771 °C. For a time, the non-magnetic (actually,
paramagnetic) form of α-iron, existing in α-iron above 771 °C, was called
β-iron, but
metallurgists are more concerned with structure than magnetism, so the term, "β-iron," is now
obsolete.
Marching up the Greek alphabet to γ gets us to
γ-iron, also called Austenite, named after
British metallurgist,
William Chandler Roberts-Austen (1843-1902), that exists from 910-1394 °C.
γ-iron has the
face-centered cubic crystal structure. Above 1394 °C, there's
δ-iron with the
body-centered cubic crystal structure. At
normal pressure, the δ-iron melts at 1538 °C.
If we're willing to subject iron to very high pressure, on the order of 100,000 bar, it's possible to form
ε-iron, also called
hexaferrum. ε-iron has the
hexagonal close-pack crystal structure, consistent with the concept of pressing the iron into a
dense state (see table).
While ε-iron is not
technologically useful, it might be an important phase in
geology. The pressure at
Earth's core is estimated to be about 1,500,000-3,000,000 bar, well above the pressure at which ε-iron exists at r
room temperature. ε-iron might even exist at the much higher temperature of Earth's core, about 3000 °C, at those pressures. Proving this
experimentally, however, might be as difficult as a
journey to Earth's core. The
triple point between the α-, γ- and ε- phases of iron has been
calculated to be at a temperature of 770
K (497 °C) and a pressure of 11
GPa.
Crystalline materials are not the only materials used to construct our
modern culture.
Polymers, formed from
organic molecules are ubiquitous materials; and, while
some of these can be up to 80% crystalline, non-crystalline polymers, such as the
synthetic fiber,
nylon, have desirable
mechanical properties. Polymers are formed from smaller organic molecules called
monomers that link together into long,
linear chains through
carbon-carbon bonding.
The atoms of organic molecules, such as the monomers from which polymers are made, can arrange themselves differently in
space, just like the iron atoms in crystalline iron. There can be several forms of
molecular configuration, called
enantiomers, that are
mirror images of each other and are characterized by how they
rotate the
plane of polarization of
transmitted light. Rotation
clockwise in the
propagation direction marks the molecule as dextro-rotary (d-), and levo-rotary (l-) if it's
counter-clockwise.
The d- and l-
nomenclature comes from the
Latin words for
right,
dexter, and
left,
laevus. Such molecules might have in addition to the d- and l- forms a configuration in which there is no
optical activity, called
meso-rotary. Quite importantly, just one
enantiomorph of some
pharmaceuticals is useful, the other being non-active, and sometimes
toxic.
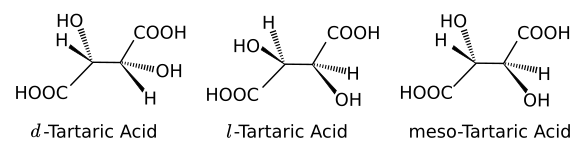
Three stereochemical configuration of tartaric acid. (Drawn using Inkscape.)
Organic molecules don't exist just as linear chains of carbon atoms with other elements attached, but also as
rings. We have the examples of
benzene, C
6H
6, and its close cousin,
Pyridine, C
5H
5N. Such an unexpected
ring structure for molecules was first published in 1865 by
German chemist,
Friedrich August Kekulé (1829-1896), as the structure for benzene.
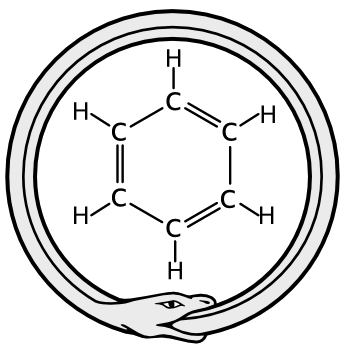
The structure of benzene, C6H6.
By Kekulé's own account, this idea came to him in a daydream of the ouroboros, the image of a snake biting its own tail.
(Ouroboros image and the benzene structural diagram, via Wikimedia Commons)
![]()
A team of
physicists from the
University of Vienna (Vienna, Austria) and the
Johannes Gutenberg University Mainz (Mainz, Germany) have researched a method to separate
chemically identical linear
macromolecules from ring macromolecules.[1-2] They used
computer dynamics simulations that incorporate
hydrodynamic effects to design
microfluidic channels to do this
separation of polymers in
dilute solutions.[1-2] At this time, the technique still requires experimental verification.
Since some of their properties are so different, separation of chemically identical linear macromolecules from ring macromolecules could be important in some applications. Circular molecules lack ends, so they are more resistant to
degradation, and also less likely to become
entangled. Such properties of ring molecules are important in
Nature since they enhance the resilience of
DNA and
RNA against degradation.[2]
The approach to separation taken by the
research team is based on the idea that linear and ring molecules, and mixtures thereof, can
flow differently under the proper conditions.[2] There is essentially no difference in flow rate when these molecules flow in channels with smooth and
repulsive walls.[1] If the channel walls are
studded with
attractive spots arranged on lines
parallel to the flow, ring polymers have an
order of magnitude higher
velocity than linear chains.[1] This effect is more pronounced when the polymer molecules are more
rigid.[1] The linear chains are immobilized on these spots, but the ring molecules can
roll along them (see figure).[2] The project was funded by the
European Union's Horizon 2020 research and innovation program.[2]
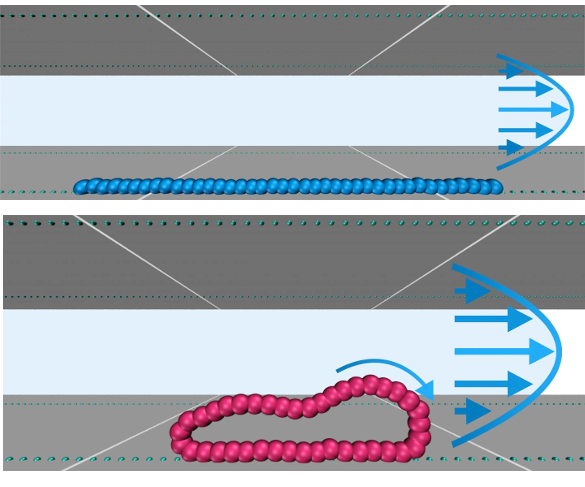
Polymer linear chain (top) and ring (bottom) flowing in a microchannel studded with attractive points (green dots). Also shown is the flow profile and the rolling motion of a polymer ring. (Top image and bottom image, copyright Lisa Weiss, University of Vienna.)
References:
- Lisa B. Weiss, Arash Nikoubashman, and Christos N. Likos, "Topology-Sensitive Microfluidic Filter for Polymers of Varying Stiffness," ACS Macro Lett., vol. 6 (December 5, 2017), pp 1426-1431, DOI: 10.1021/acsmacrolett.7b00768.
- Nanomaterials: How to separate linear and ring-shaped molecules, University of Vienna Press Release, December 6, 2017.
Linked Keywords: Iron; atom; chemical reaction; chemically; material; crystal structure; materials properties; allotropes of iron; heat; heating; atmospheric pressure; bar (unit); convention (norm); Greek alphabet; Greek letter; alphabet; embarrassment; embarrassed; scientist; chemical element; phase diagram of pure iron; phase diagram; iron; Wikimedia Commons; Daniele Pugliesi; phase; hand; ferrite (iron); α-iron; body-centered cubic; crystal structure; Celsius; °C; magnetism; magnetic; Curie temperature; paramagnetism; paramagnetic; beta ferrite; β-iron; metallurgy; metallurgist; obsolescence; obsolete; Austenite; γ-iron; Great Britain; British; William Chandler Roberts-Austen (1843-1902); face-centered cubic; delta iron; δ-iron; atmospheric pressure; normal pressure; hexaferrum; ε-iron; hexagonal close-pack; density; dense; packing fraction; diamond cubic; technology; technological; geology; inner core; Earth's core; room temperature; experiment; experimental; Journey to the Center of the Earth; triple point; calculation; calculated; kelvin; pascal; GPa; crystal; crystalline; modern culture; polymer; organic molecule; crystallization of polymers; synthetic fiber; nylon; mechanics; mechanical; monomer; linearity; linear; long chain molecule; chain; carbon-carbon bond; space; molecular configuration; enantiomer; mirror image; rotation; rotate<; plane of polarization; transmittance; transmitted light; clockwise; Poynting vector; propagation direction; counter-clockwise; nomenclature; Latin; relative direction; right; left; optical rotation; optical activity; meso compound; meso-rotary; chirality; enantiomorph; pharmaceutical drug; toxicity; toxic; stereochemistry; stereochemical; tartaric acid; Inkscape; cyclic compound; ring; benzene; pyridine; German; chemist; Friedrich August Kekulé (1829-1896); Kekulé's dream; idea; daydream; ouroboros; snake; biting; tail; physicist; University of Vienna (Vienna, Austria); Johannes Gutenberg University Mainz (Mainz, Germany); chemical reaction; chemically; macromolecule; analytical dynamics; computer dynamics simulation; fluid dynamics; hydrodynamic; microfluidic channel; separation process; concentration; dilute; solution; chemical decomposition; degradation; knot; entangle; Nature; DNA; RNA; research; flow; electrostatic; repulsive; studded; attractive; parallel; order of magnitude; velocity; stiffness; rigid; rolling; roll; European Union; Horizon 2020 research and innovation program.