Giant Magnetocaloric Effect
September 10, 2018
While it's common for
experimental physicists to
invent something that's
patentable, this is typically not true for
theoretical physicists. However, it sometimes happens. The
Hungarian-
German-
American physicist,
Leo Szilard (1898-1964), and
Italian-American physicist,
Enrico Fermi (1901-1954), applied for a patent on the
nuclear reactor in 1944. The 58 page patent,
US patent no. 2,708,656, wasn't issued until a
decade later for
security reasons.[1]
Another example is
Albert Einstein (1879-1955), who teamed with Szilard on the design of a
refrigerator. As I wrote in a
previous article (Ammonia Synthesis, March 6, 2017),
ammonia was used as a
refrigerant in
household refrigerators in the early
20th century. It was a bad choice, based on its
toxicity, but a good choice based on its
thermodynamics. Ammonia has a low
boiling point (-33.34
°C, -28.0
°F) and high
enthaly of vaporization (23.35 kJ/mol, 5.56 kcal/mol at its boiling point).
Methyl chloride and
sulfur dioxide, likewise toxic chemicals, were also used in home refrigerators.
Einstein read how an entire
family had been
killed as they
slept by leaking refrigerator fumes.[2] Moved by this tragedy, Einstein teamed with Szilard to invent and patent the "
Einstein refrigerator."[3] This refrigerator still used the standard toxic gases, but it was safer since it had no moving parts and did not require
rotary seals (see figure). The application of the first
commercial freon (
Freon-12,
R-12, or
CFC-12) to refrigeration in the 1930s likely saved many lives; but, as happens for many wonderful
technologies, there were also
unintended consequences. In the case of freon, it was
destruction of atmospheric ozone.
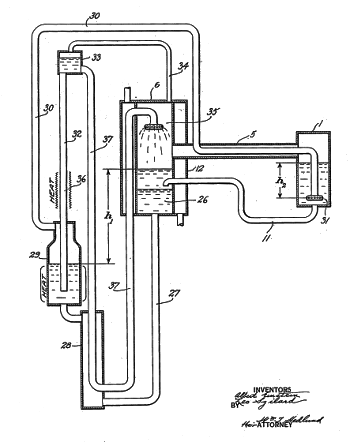
Figure from US Patent No. 1,781,541, "Refrigeration," by Albert Einstein and Leo Szilard, dated November 11, 1930.
The "Einstein refrigerator" is an absorption refrigerator in which the cooling evaporate is absorbed by another liquid, which is run through a heat exchanger to recover the refrigerant for another refrigeration cycle.
(Via Google Patents.)[3]
As most people know, Einstein was quite familiar with patents, since he was employed by the
Swiss patent office early in his career. Also, Jacob Einstein, his
uncle, held seven patents, many of which concerned
electric arc lamps.[4] Einstein was issued 19 patents in his lifetime. most of which were with
collaborators such as Szilard, and many of these patents are the same invention patented in different countries.[4] One interesting invention is U.S. patent no. 1,017,566, "Design of a
blouse," issued in 1936.[5]

Albert Einstein's patent for a blouse, issued on October 27, 1936, was a design patent, not a utility patent.
My conjecture is that Einstein did this as a respite from more ponderous thought, just as one of today's scientists will view an entertaining video clip on his computer screen in the middle of a serious calculation.
(Via Google Patents.)[5]
![]()
Refrigeration is still being
researched, and one important research area is
magnetic refrigeration. This isn't about the best
magnets to affix a
child's artwork to a refrigerator door; rather, it's about a means of
solid-state cooling using a magnetic material in a
magnetic field using what's termed the
magnetocaloric effect. I wrote about the magnetocaloric effect in an
earlier article (Magnetic Refrigeration, September 3, 2014).
In 1881,
German physicist,
Emil Warburg, discovered that
iron would cool upon application of a magnetic field. His iron specimen cooled about a degree
Celsius when subjected to an applied field of one
tesla (10,000
gauss), which is about 20,000 times the strength of
Earth's magnetic field. While Warburg's experiment showed what is now called the magnetocaloric effect, there are arguments that the effect was only elucidated in 1917 by
Pierre Weiss (1865-1940) and
Auguste Piccard (1884-1962).[6] Weiss' name is associated with the important
Curie–Weiss law.
The magnetocaloric effect uses
entropy as a means of extracting
heat from a substance. The magnetic field aligns the
magnetic moments of the
atoms in a
solid, which allows a little more heat to be extracted from the material. This results in a
temperature change, and the temperature can be lowered again by repeating the cycle. The
thermodynamic cycle associated with the magnetocaloric effect can be seen in the figure. Magnetic refrigeration was first used as a means of cooling small
volumes to temperatures near
absolute zero. This method was developed by
University of California, Berkeley,
chemist and
Nobel laureate,
William Giauque (1895-1982).
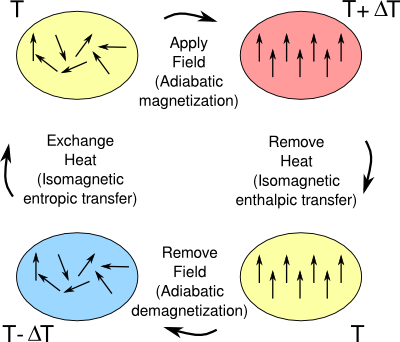
The thermodynamic cycle of a magnetic refrigerator.
(Created using Inkscape. Click for a larger image.)
What
material properties result in a good material for magnetic refrigeration? A cursory glance at the following
equation that relates the change in temperature
T to the change in magnetic field
H tells the entire story.
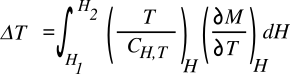
![]()
In this case, instead of the usual
heat capacities at constant volume (C
v) or constant
pressure (C
p), the
integral contains an expression for the particular heat capacity at each temperature and magnetic field (C
H,T). Aside from the obvious fact that larger applied magnetic fields will give a greater temperature change, we see that materials with smaller heat capacity are better, and also that large temperature changes come from materials that have a large change in magnetization
M with temperature.
This explains why
gadolinium is the material used in the many
YouTube demonstrations of the magnetocaloric effect. Gadolinium is about thirty times more expensive than iron, but it's commonly available. As can be seen in the graph, gadolinium has a
Curie temperature quite near
room temperature, so it has a large dM/dT at room temperature.
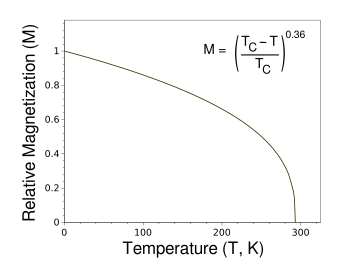
Ideal magnetization curve of gadolinium as a function of temperature.
The function shown gives the temperature dependence of magnetization of a ferromagnetic material according the the mean-field approximation.
(Created using Gnumeric.)
While pure gadolinium will function in a magnetic refrigerator, some
compounds of gadolinium perform better. These include Gd
85Er
15, Gd
5(Si
2Ge
2), and compounds of the general
formula, Gd
5(Si
xGe
1−x)
4.[6] Now, a research team from the
U.S. Department of Energy's Ames Laboratory (Ames, Iowa),
Iowa State University (Ames, Iowa), and the
European Synchrotron Radiation Facility (Grenoble, France) has discovered a giant magnetocaloric effect in the
rare earth intermetallic compound, Eu
2In.[7-8] The magnetocaloric effect in this compound arises from a novel
discontinuous magnetoelastic transition, and the discovery could lead to even better magnetocaloric materials.[7-8]
The
first-order magnetic transition is marked by the presence of a
latent heat of transformation, and few materials are known to have magnetoelastic first-order magnetic transitions at room temperature when at least one phase has a large magnetization.[7] Eu
2In has such a transition at low magnetic fields and almost no
hysteresis.[7-8]
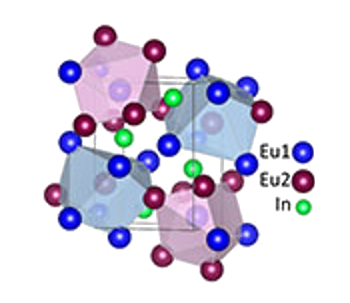
Structure of Eu2In.
The two distinct europium lattice sites are important to this compound's functioning as an excellent magnetocaloric material.
(Ames Laboratory image.[9])
X-ray absorption and
magnetic circular dichroism experiments were conducted at the European Synchrotron Radiation Facility to determine the
physical mechanism of the transformation.[8] Says
Durga Paudyal, a scientist at Ames and an
author of the
paper that describes this research, "The magnetic phase transition can be explained by an unusual
exchange of electrons between the two
elements in the compound, with indium electronic states overlapping with those of europium."[8] There is an electron transfer between a
5d electron state of europium and a
4p electron state of indium.[7]
Research team member
Vitalij Pecharsky, who holds a joint appointment at Iowa State and Ames, explains the importance of the discovery the unusual mechanism of the compound's change in magnetic state.
"Now that we have seen this mechanism and are able to explain how it works, we can use this knowledge to look for similar but better materials, one that can be used in future applications like magnetic refrigeration."[8]
References:
- E. Fermi and L. Szilard, "Neutronic reactor," U.S. patent no. 2,708,656, May 17, 1955.
- Gene Dannen, The Einstein-Szilard Refrigerators, Scientific American, January 1997, pp. 90-95.
- Albert Einstein and Leo Szilard, "Refrigeration," US patent No. 1,781,541, November 11, 1930.
- Asis Kumar Chaudhuri, "Einstein's Patents and Inventions," arXiv, September 5, 2017.
- Albert Einstein, "Design for a blouse," U.S. design patent no. 101,756, October 27, 1936.
- Anders Smith, "Who discovered the magnetocaloric effect?" The European Physical Journal H, vol. 38, no 4 (September, 2013), pp 507-517.
- V. K. Pecharsky and K. A. Gschneidner, Jr., "Giant Magnetocaloric Effect in Gd5(Si2Ge2)," Phys. Rev. Lett., vol. 78 (June 9, 1997), Document No. 4494, DOI: http://dx.doi.org/10.1103/PhysRevLett.78.4494.
- F. Guillou, A. K. Pathak, D. Paudyal, Y. Mudryk, F. Wilhelm, A. Rogalev, and V. K. Pecharsky, "Non-hysteretic first-order phase transition with large latent heat and giant low-field magnetocaloric effect," Nature Communications, vol. 9, Article no. 2925, July 26, 2018. This is an open access article with a PDF file here.
- Unusual rare earth compound opens doorway to new class of functional materials, Ames Laboratory Press Release, July 26, 2018.
- Stinus Jeppesen, "Magnetocaloric materials," Ph.D Thesis - University of Copenhagen, October, 2008, ISBN 978-87-550-3706-9.
Linked Keywords: Experimental physics; experimental physicist; invention; invent; patent; theoretical physics; theoretical physicist; Hungary; Hungarian; Germany; German; United States; American; Leo Szilard (1898-1964); Italy; Italian; Enrico Fermi (1901-1954); nuclear reactor; US patent no. 2,708,656; decade; national security; Albert Einstein (1879-1955); refrigerator; ammonia; refrigerant; household; 20th century; toxic; toxicity; thermodynamics; boiling point; Celsius; °C; Fahrenheit; °F; enthaly of vaporization; methyl chloride; sulfur dioxide; family; death; kill; sleep; Einstein refrigerator; radial shaft seal; rotary seal; commerce; commercial; freon; technology; unintended consequences; ozone depletion; destruction of atmospheric ozone; absorption refrigerator; evaporation; evaporate; liquid; heat exchanger; thermodynamic cycle; Google Patents; Swiss Federal Institute of Intellectual Property; Swiss patent office; uncle; electric arc lamp; collaboration; collaborator; blouse; design patent; utility patent; conjecture; scientist; video clip; computer monitor; computer screen; calculation; research; magnetic refrigeration; magnet; child; art; artwork; solid-state; magnetic field; magnetocaloric effect; Germany; German; physicist; Emil Warburg; iron; tesla; gauss; Earth's magnetic field; Pierre Weiss (1865-1940); Auguste Piccard (1884-1962); Curie–Weiss law; entropy; heat; magnetic moment; atom; solid; temperature; volume; absolute zero; University of California, Berkeley; chemist; Nobel laureate; William Giauque (1895-1982); Inkscape; material property; equation; heat capacity; pressure; integral; gadolinium; YouTube; Curie temperature; room temperature; magnetization; function (mathematics); ferromagnetic material; mean field theory; mean-field approximation; Gnumeric; chemical compound; empirical formula; U.S. Department of Energy; Ames Laboratory (Ames, Iowa); Iowa State University (Ames, Iowa); European Synchrotron Radiation Facility (Grenoble, France); rare earth element; intermetallic compound; discontinuity; discontinuous; inverse magnetostrictive effect; magnetoelastic; phase transition; first-order magnetic transition; latent heat of transformation; hysteresis; crystal structure; europium; lattice site; X-ray; absorption spectroscopy; magnetic circular dichroism; experiment; physical mechanism; Durga Paudyal; author; scientific literature; paper; exchange interaction; exchange of electrons; chemical element; electron shell; 5d electron; 4p electron; Vitalij Pecharsky; E. Fermi and L. Szilard, "Neutronic reactor," U.S. patent no. 2,708,656, May 17, 1955; Albert Einstein and Leo Szilard, "Refrigeration," US patent No. 1,781,541, November 11, 1930; Albert Einstein, "Design for a blouse," U.S. design patent no. 101,756, October 27, 1936.