Ultra-Pure Green Light
November 20, 2017
As I look at the world around me in this heavily
forested area of
Northern New Jersey, I see mostly
green.
Vegetation, from
tree leaves to the blades of
grass on my
lawn, is green. The reason for this is that green light is
not absorbed by this vegetation, so it's
reflected. The light-absorbing
pigments in vegetation,
Chlorophyll a and
Chlorophyll b, extract
energy from the
Sun at short and long
visible wavelengths, but they leave a gap in
absorbance centered around green light (see
graph).
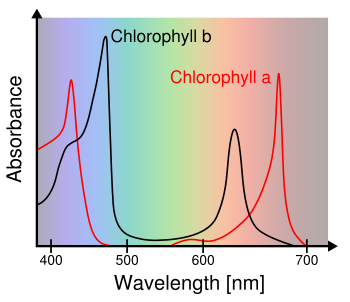
Chlorophyll a and Chlorophyll b absorbance spectra.
(Wikimedia Commons image, modified, by M0tty.)
(Click for larger image.)
Unless you're lost in the
woods,
green is the preferred color choice for an object you want to see. As the graph below illustrates, the
human eye sees green objects much better than
red or
blue objects. This
curve was developed by asking
study participants to equalize the perceived
intensity of various pairs of colored lights, and then doing a
mathematical analysis of these
data.
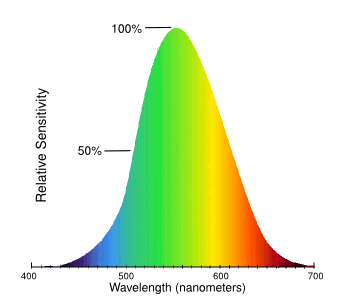
As can be seen from this graph, the color sensitivity of the human eye peaks at green.
The human eye can distinguish about 10 million different colors.
(Modified Wikimedia Commons image.)
As most people know, green is one one of the
three principal colors used to render images on
television,
cellphone, and
computer displays, the others being red and blue. Mixing of these primary colors enables a representation of many colors. In a typical system in which the intensity of each of these colors is represented by an
eight bit value, there are 2
24 (16,777,216) possible colors. Since the human eye can distinguish about 10 million different colors, this is quite adequate; provided, however, that the red, green, and blue colors are of sufficient
purity.
The specific wavelengths of the red, green, and blue colors determine how many of the colors perceived by humans can be represented. Based on
experiments on
color vision in humans performed in the 1920s, the
International Commission on Illumination (CIE) created a "
color space" relating perceived color to the wavelengths of light. Although created many years ago, this
CIE 1931 color space, is still used, and I've used it in my own work on
phosphor materials. If the wavelengths and purity of your red, green, and blue light sources are different from the limiting values for this color space, the area of represented colors will shrink.
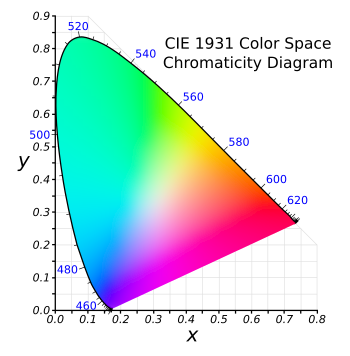
The CIE 1931 color space chromaticity diagram.
As can be seen from the figure, the wavelengths are specified at the periphery, with red, green, and blue establishing the limits of possible colors.
White light is naturally placed at the center; and, interestingly, you can get white light with a proper choice of just two color wavelengths.[1]
(Modified Wikimedia Commons image by "BenRG.")
(Click for larger image.)
Green light may sit in the middle of the visible spectrum, but it's more difficult to produce green light than red or blue light. The first viable
light emitting diodes (LEDs) were
infrared and red, but producing high
efficiency green LEDs is difficult. Blue LEDs arrived at a much later time, but high efficiency blue LEDs are now commonplace. In fact, the same
technology that gives us blue LEDs also produces
ultraviolet (UV) LEDs, and UV-LEDs can excite phosphors to produce green light.
There are quite a few green
lasers. The common
helium–neon red light
gas lasers that were common in
supermarket barcode scanners before the arrival of
semiconductor lasers can also be tuned to emit green light at 543.5
nanometer (nm), but at low efficiency.
Argon ion lasers emit green light at 514.5 nm, also at low efficiency.
Copper vapor can be used as a laser at 510.6 nm.
If the light intensity is large enough, as from a high powered laser, it's possible to
"double" the frequency of its light (halve its wavelength) by passing it through a
nonlinear optical material. In this manner, it's possible to double the 1064-nm infrared light from a
neodymium-YAG or
Neodymium-doped yttrium orthovanadate (Nd:YVO
4) laser to produce 532 nm green light. By a different physical process called
upconversion you can "add" the energy of two infrared
photons together to get a green light photon. In this fashion, you can cause the light of an
erbium-doped fiber laser to upconvert to 550 nm green light.[2]
The colors represented by the chromaticity diagram presume that the red, green, and blue light sources are
monochromatic; that is, the light emission is concentrated at one particular wavelength. While this is true for laser light, most light sources emit light over a range of wavelengths centered around their principal wavelength (see figure), and this reduces the number of possible colors that a display can render, which is called the
color gamut. This is clearly evident, since it's obvious that red, green and blue light sources that are so wide that they encompass the entire
visible light spectrum (white light) could not render any colors.
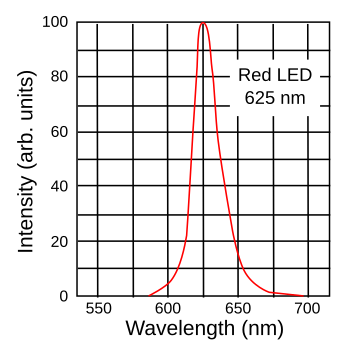
Spectrum of a red light emitting diode (LED).
This particular type of LED is an AlGaInP/GaAs diode.
(Created using Inkscape.)
One of the
research objectives in the creation of light sources is to make them as monochromatic as possible. A research team from
ETH Zürich (Zürich, Switzerland), the
Seoul National University (Seoul, Korea), the
Pohang University of Science and Technology (POSTECH, Pohang, Republic of Korea), and
Yuan Ze University (Taiwan) have produced a new type of light-emitting diode that produces ultra-pure green light.[3-4] This LED is based on
nanoscale perovskite, a material that's typically used in the reverse process of turning light into
electricity in
solar cells.[4]
The motivation for this research is to enable the next generation of higher quality color displays. While ultra-pure red and blue light sources have been developed, ultra-pure green is still a problem.[4] That's because the eye, being more sensitive to green light, can perceive more intermediary green
hues than red or blue hues. Says
Sudhir Kumar, co-lead
author of the
paper reporting this research, "This makes the technical production of ultra-pure green very complex, which creates challenges for us when it comes to developing technology and materials."[4]
The green light objective was set in 2012 by the
International Telecommunication Union (ITU), which published its
ITU-R Recommendation BT.2020 that defines the important properties of
ultra-high-definition television (UHDTV), including a wide
color gamut.[4] Ultra-pure green light is required to cover at least 95% of the CIE 1931 color space.[3] Present generation television displays cover on average only about 75% of this space, with none exceeding 80%.[4]
The LED is built from a nanoscale layer of
spin-coated,
colloidal,
two-dimensional,
formamidinium lead bromide (FAPbBr
3) hybrid perovskite that exhibits a high
photoluminescence quantum yield of about 92%.[3] The perovskite layer is just 4.8 nanometers thick.[4] As an added feature, this layer is
flexible with a
bend radius of 2
millimeters.[3] The processing is performed at
room temperature. says
Jakub Jagielski, the other co-lead author of the report, "Because we were able to realize the entire process at room temperature, we've opened up opportunities for the simple,
low-cost industrial production of ultra-green light-emitting diodes in the future."[4]
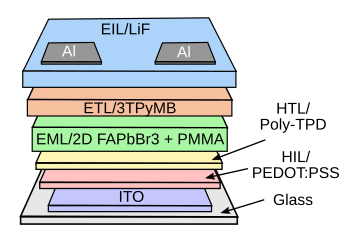
"Ultra" comes at the cost of complexity.
This is the structure of the ultra-green perovskite LED.
(Created using Inkscape.)[3]
With reference to the CIE 1931 color space chromaticity diagram, above, the color coordinates of this ultra-green light source are (0.168, 0.773).[3] Unfortunately, the efficiency is low at just 13.02
candelas/
ampere.[3] A candela is about the light emitted by a
candle. The ultra-thin light-emitting diodes are as bendable as a sheet of
paper, so they might be produced inexpensively by a
roll-to-roll process.[4]
Light sources for television displays have efficiencies of 5-10%, while the ultra-green LED has a 3% conversion of electrical energy to light.[4] Another potential problem for commercialization is the need to fix the perovskite layer at exactly 4.8 nanometers.[4] The device
lifetime is presently just a couple of hours.[4]
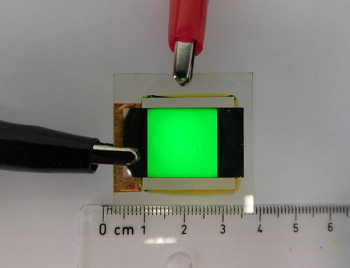
Demonstration of the ultra-green LED.
(ETH-Zürich image.)
![]()
References:
- Birger Boldt, "Method and apparatus for color synthesis," U.S. Patent No. 4,740,059, April 26, 1988.
- Web Site of RP Photonics Consulting GmbH by Dr. Rüdiger Paschotta.
- Sudhir Kumar, Jakub Jagielski, Nikolaos Kallikounis, Young-Hoon Kim, Christoph Wolf, Florian Jenny, Tian Tian, Corinne J. Hofer, Yu-Cheng Chiu, Wendelin J. Stark, Tae-Woo Lee, and Chih-Jen Shih, "Ultrapure Green Light-Emitting Diodes Using Two-Dimensional Formamidinium Perovskites: Achieving Recommendation 2020 Color Coordinates," Nano Letters, vol. 17, no. 9 (September 13, 2017), pp 5277–5284, DOI: 10.1021/acs.nanolett.7b01544.
- Florian Meyer, "Green light for ultra-fine display colours," ETH Zurich Press Release, September 6, 2017.
Linked Keywords: Forest; forested; Northern New Jersey; green; vegetation; tree; leaf; leaves; grass; lawn; reflection (physics); reflected; pigment; Chlorophyll a; Chlorophyll b; energy; Sun; visible spectrum; wavelength; absorption (electromagnetic radiation); absorbance; Cartesian coordinate system; graph; Wikimedia Commons; M0tty; woods; human eye; color vision; red; blue; curve; clinical trial; study participant; intensity (physics); mathematics; mathematical; analysis; data; color sensitivity; RGB color model; three principal colors; television set; mobile phone; cellphone; computer monitor; computer display; 8-bit; eight bit; colorfulness; color purity; experiment; color vision in humans; International Commission on Illumination (CIE); color space; CIE 1931 color space; phosphor; material; CIE 1931 color space chromaticity diagram; wavelength; light emitting diode; infrared; energy conversion efficiency; technology; ultraviolet; laser; helium–neon; gas laser; supermarket; barcode reader; barcode scanner; laser diode; semiconductor laser; nanometer; argon ion laser; copper vapor laser; second-harmonic generation; frequency doubling; nonlinear optics; nonlinear optical material; Nd:YAG laser; neodymium-YAG; Neodymium-doped yttrium orthovanadate; photon upconversion; photon; erbium-doped fiber laser; monochrome; monochromatic; color gamut; visible light spectrum; electromagnetic spectrum; light emitting diode; aluminium gallium indium phosphide; AlGaInP; gallium arsenide; GaAs; Inkscape; research; ETH Zürich (Zürich, Switzerland); Seoul National University (Seoul, Korea); Pohang University of Science and Technology (POSTECH, Pohang, Republic of Korea); Yuan Ze University (Taiwan); nanoscopic scale; nanoscale; perovskite; electricity; solar cell; hue; Sudhir Kumar; author; scientific literature; paper; International Telecommunication Union; ITU-R Recommendation BT.2020; ultra-high-definition television; color gamut; spin coating; spin-coated; colloid; colloidal; two-dimensional; amidine; formamidinium; lead bromide; photoluminescence; quantum yield; deflection; flexible; bend radius; millimeter; room temperature; Jakub Jagielski; low-cost; industrial production; complexity; candela; ampere; candle; paper; roll-to-roll process; lifetime.