Rise and Fall of Coal
February 4, 2016
The
Industrial Revolution began in the
United Kingdom, with
steam engines fueled by
coal. It is, therefore, significant that the last
deep-pit mine in the UK, located in
Kellingley Colliery,
Beal, North Yorkshire, closed on December 18, 2015.[1] The closing ended
employment for 450
coal miners,[1] and it was the final
coffin nail for
Britain's Industrial revolution.

Coal mining.
Whoever said that "hard work never killed anyone," didn't study coal miners.
(Illustration from an 1871 Swedish schoolbook, via Wikimedia Commons.)
Coal mining in Britain goes back as far as the
Roman Empire. The Romans didn't use coal as a
fuel, since it would have been too hard to
transport. Instead, they
carved it to make
jewelry;[1] and, it's mentioned in
Pliny's Natural History as an
ophthalmic medication.[2] Much earlier than Pliny,
Theophrastus (c. 371 - c. 287 BC) wrote about coal in his
treatise, "
On Stones" (Περι Λιθων) (see figure).

Section 16 of "On Stones" by Theophrastus. The passage reads, "Among the substances that are dug up because they are useful, those known simply as coals are made of earth, and they are set on fire and burnt like charcoal. They are found in Liguria, where amber also occurs, and in Elis as one goes by the mountain road to Olympia; and they are actually used by workers in metals." (Translation by E. R. Caley and J. C. Richards.[3]
Coal was big business by the end of the
19th century. The first million
pound (£) contract in
history involved the supply of coal.[1] Coal production in the
United States peaked in the early
20th century, my
maternal grandparents had a coal-fired
furnace, and I enjoyed recovering small lumps of coal from their
cellar. The following
graph of
anthracite coal production in
Pennsylvania illustrates the decline of coal in the United States.[4]
China's coal production peaked in 2013 at about 3,700 million
metric tons per year.
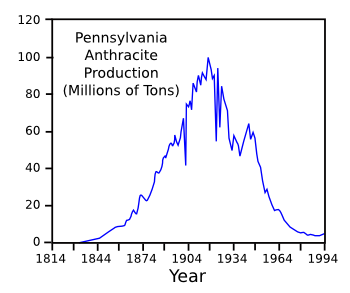
For whom the bell (curve) tolls.
Anthracite mined in Pennsylvania peaked around 1920, and it's declined to nearly zero today.
(Data from United States Geological Survey Circular 1147.)
Science and
technology arise to support major
industries, and this was the case, also, for coal mining. Coal miners require
illumination, which was provided by either
candles or
oil lamps in the days before
electricity. Since coal contains
volatile organic compounds, such
flammable gases, called firedamp, could be ignited by a flame. The
chemist,
Humphry Davy (1778 - 1829), who's famous for discovering many
chemical elements through the
electrolysis of
molten salts, invented a
safe lamp.
This "safety lamp" was just a standard
wick-type oil lamp fitted with a
wire mesh between the flame and the
atmosphere. The flame is prevented from passing through the mesh, so it will not ignite the outside gases. The lamp was not completely safe, since errant gusts of air would render the screen protection ineffective, and the flame would pass through. Under normal operation, the mesh would
glow when high
concentrations of flammable gas were present. Some mines would use a Davy lamp as an indicator of when it was safe to use other lamps.
.jpg)
"Careful, m'lad. That's one way to burn your finger!
Shades of Johnny Tremain! With the period dress, this reminds me of the book I was forced to read while in elementary school. Johnny's burned hand is a plot point in that book. Even without werewolves and vampires, young-adult fiction was still macabre.
(Illustration from the 1878 book, "The story of Sir Humphrey Davy and the invention of the safety-lamp," Published by T. Nelson, London, and held in the Harold B. Lee Library of Brigham Young University, via Wikimedia Commons.)
As most
materials dug from the ground, coal is far from the
pure carbon form we desire.
Anthracite, the best
grade of coal, has the following approximate
composition:
mineral ash ≤ 20%; volatiles ≤ 10%;
hydrogen ≤ 3.75%;
oxygen ≤ 2.5%;
sulfur ~1%; carbon = balance. Anthracite has a
combustion enthalpy of about 30,000
kJ/
kg. Aside from the obvious problem in release of
carbon dioxide into the atmosphere through combustion,
sulfur dioxide emission produces
acid rain.
Simplified structural formula of hard coal.
Fortunately, I was never subjected to an undergraduate organic chemistry course. My wife, however, excelled in organic chemistry.
(Illustration by Karol Głąb, via Via Wikimedia Commons.)
If it wasn't for some very specific features of the
evolution of life on
Earth, we would not have had coal. As a consequence, our technological growth would have been limited, and you would not be reading this article on the
Internet. This brings up the possibility that most
intelligent extraterrestrial species would not develop technology as rapidly as we
Earthlings.
As I wrote in an
earlier article (Coal, July 23, 2012), coal is the
fossilized remains of
lignin, a complex
polymer that's a part of the
cell walls of
plants that lived about 300-360 million years ago in the aptly named
Carboniferous period. Coal is found today, since vast quantities of lignin were produced in that period as a
solar energy reservoir.
Evolutionary forces also put an end to the Carboniferous period, defining the height and depth of coal
seams. As discovered in a 2012 study,
white rot fungi evolved at the end of the Carboniferous with the capacity to
digest lignin, thus ending the sixty-million year span of coal deposition by destroying the accumulation of
woody debris that fossilized as coal.[5-7]

A lump of anthracite coal
(An image from the "Minerals in Your World" project, a collaboration of the United States Geological Survey and the Mineral Information Institute, via Wikimedia Commons.)
![]()
References:
- Steffan Morgan, "Britain's last lump of coal," Pittsburgh Post-Gazette, January 3, 2016.
- Pliny, Natural History, Book 36, Chapter 38, John Bostock and H.T. Riley, Trans., (London: Henry G. Bohn, 1857), p. 364.
- Theophrastus, "On Stones - A Modern Edition with Greek Text, Translation, Introduction, and Commentary, E. R. Caley and J. C. Richards, Eds., The Ohio State University Press, 1956.
- Robert C. Milici and Elisabeth V. M. Campbell, "The Use of Historical Production Data to Predict Future Coal Production Rates," U.S. Geological Survey Circular 1147, September 17, 1997.
- Study on Fungi Evolution Answers Questions About Ancient Coal Formation and May Help Advance Future Biofuels Production, NSF Press Release 12-117, June 28, 2012.
- Chris Todd Hittinger, "Endless Rots Most Beautiful," Science, vol. 336 no. 6089 (June 29, 2012) pp. 1649-1650.
- David S. Hibbett, et al., "The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes," Science, vol. 336 no. 6089 (June 29, 2012) pp. 1715-1719.
Permanent Link to this article
Linked Keywords: Industrial Revolution; United Kingdom; steam engine; coal; deep-pit mine; Kellingley Colliery; Beal, North Yorkshire; employment; coal miner; coffin; nail; Britain; coal mining; coal miner; Sweden; Swedish; textbook; schoolbook; Wikimedia Commons; Roman Empire; fossil fuel; transport; carving; carved; jewelry; Pliny the Elder; Natural History; ophthalmology; ophthalmic; medicine; medication; Theophrastus; treatise; On Stones; Translation by E. R. Caley and J. C. Richards; 19th century; pound sterling; contract; history; United States; 20th century; maternal grandparent; furnace; basement; cellar; Cartesian coordinate system; graph; anthracite coal; Pennsylvania; China's coal production; metric ton; bell; normal distribution; bell curve; United States Geological Survey Circular 1147; science; technology; industry; lighting; illumination; candle; oil lamp; electricity; volatile organic compound; flammability; flammable; ga; chemist; Humphry Davy; chemical element; electrolysis; melting; molten; salt; safety; candle wick; wire mesh; atmosphere of Earth; incandescence; glow; concentration; ghost; shade; Johnny Tremain; fashion; period dress; book; elementary school; burn; hand; plot point; werewolf; werewolves; vampire; young-adult fiction; macabre; Harold B. Lee Library; Brigham Young University; material; chemical element; pure; carbon; ore; grade; composition; mineral; ash; hydrogen; oxygen; sulfur; combustion; enthalpy; joule; kJ; kilogram; kg; carbon dioxide; sulfur dioxide; acid rain; structural formula; undergraduate education; organic chemistry; course; wife; Karol Głąb; evolution of life; Earth; Internet; intelligent extraterrestrial species; Earthling; fossil; fossilized; lignin; polymer; cell wall; plant; Carboniferous; geologic period; solar energy; stratum; seam; wood-decay fungus; white rot fungi; digestion; digest; woody debris.