Portobello Power
October 26, 2015
While
materials scientists pride themselves on their development of many
technologically useful
materials, they're often outclassed by
nature. At the time I was doing
research in
superconductivity, our
laboratory was using so much
liquid helium that it was
economical to have our own
liquefaction plant. The helium liquefier that we used had
gaskets made from natural
leather, since it was an inexpensive material capable of maintaining
flexibility at
low temperatures.
Integrated circuit manufacturers have used
apricot pits as an
abrasive for
deflashing plastic IC packages. Apricot pits, as well as
walnut shells, are ideal media for this application, since they have the proper
mechanical properties, they're
non-toxic, and they don't produce problematic
mineral dust.

A raft of apricot, looking like atoms on the surface of a cubic crystal.
Turkey is the foremost apricot producing country in the world. Its annual production of about 800,000 metric tons far outstrips the US annual production of about 55,000 metric tons.
(United States Department of Agriculture, Agricultural Research Service, photo by Scott Bauer, via Wikimedia Commons.)
There's another reason to love apricots. Along with
mulberries and other
natural products, they're mentioned as an
aphrodisiac in
Shakespeare's,
A Midsummer Night's Dream.[1]
Be kind and courteous to this gentleman;
Hop in his walks and gambol in his eyes;
Feed him with apricocks and dewberries,
With purple grapes, green figs, and mulberries;
The honey-bags steal from the humble-bees,
And for night-tapers crop their waxen thighs
And light them at the fiery glow-worm's eyes,
To have my love to bed and to arise;
Mushrooms are a common and inexpensive natural product. Although my
colleague corporate scientists and I used to
joke about the
mushroom management of our companies, we didn't see anything technologically useful about mushrooms as a material source. Now, materials scientists from the
University of California, Riverside, have found that pyrolyzed carbon derived from
Portobello mushrooms (Agaricus bisporus) acts as an excellent
anode material for
lithium-ion batteries.[2-3]

Underside of the cap (pileus) of a Portobello mushroom (Agaricus bisporus).
(Wikimedia Commons photo by Lisa Redfern.)
First, a word about
nomenclature. In my reading of the references for this article, I found that people use alternative spellings for "Portobello mushroom." As shown in the figure, a
Google Search finds 927,000
websites in which the mushroom is spelled as "Portobello," about half as many using "Portabella," and a considerable number using two other spellings. This must be how
written languages without
vowels developed. The preferred spelling is "Portobello."
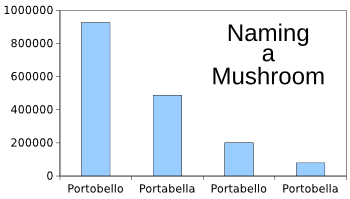
Mushroom spelling by popular vote.
(Google Search results of the number of websites using various spellings, graphed using Gnumeric.)
Carbon is a typical anode material for lithium-ion batteries, and its usual source is
synthetic graphite produced by
pyrolysis of
sucrose.[2] The
porosity of the graphite, which results in a high
surface area, is important to the anode application, so a subsequent treatment is required to "
activate" the carbon. The usual activation process involves exposing the material to concentrated
potassium hydroxide (KOH), and then heating to create mesopores and/or micropores, and this allows a
battery capacity of more Than 1100
mA-h/
g.[2]
Treatment with a strong
base like KOH is not
environmentally friendly, and that has encouraged scientists to look for a
biomass replacement.[3]
Engineers at the University of California, Riverside, decided to look at mushroom biomass, since mushrooms have naturally high porosity. For mushrooms, this porosity is important to allow the
absorption of
water and
air. Furthermore, mushrooms have a high
concentration of
potassium salts, which would allow pore growth during use.[3]
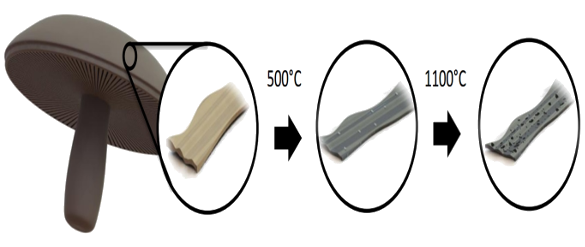
Diagram showing how carbon battery material is created from Portobello mushrooms. The ribbon-like mushroom material is heated at 500°C, where it dehydrates and mostly converts to carbon. A final heating at 1100°C results in full pyrolysis, creating hierarchically porous carbon nanoribbons. (UC Riverside illustration.)
The process for creation of carbon anode material from Portobello mushrooms, a two-stage pyrolysis, is shown in the above figure.
Oxygen-rich
organic compounds and
potassium compounds in the mushroom skins create inner void spaces in carbon nanoribbons, and the resulting material is analogous to activated carbon.[2] The pyrolysis at 900°C produces a
hierarchically porous material with pore size ranging from sub-
nanometer to tens of nanometers.[3]
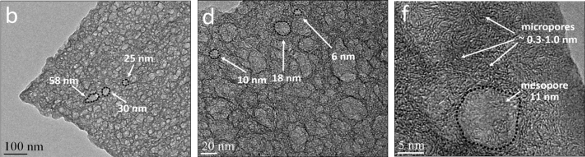
A transmission electron micrograph of hierarchically porous nanoribbons produced from Portobello mushrooms. Shown are macropores (b), mesopores (d) and worm-like micropores (f). (Portion of fig. 4 of ref. 2, licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License.)[2)]
While conventional activated carbon anodes allow the lithium
ions to fill most of the material during the first few
cycles, the battery capacity degrades after that time since electrode damage occurs. The Portobello mushroom material behaves differently. Says Brennan Campbell, a
graduate student in
Materials Science and Engineering at UC Riverside, and a
co-author of a
paper about this
research,
"With battery materials like this, future cell phones may see an increase in run time after many uses, rather than a decrease, due to apparent activation of blind pores within the carbon architectures as the cell charges and discharges over time."[3]
The Portobello mushroom anode material was shown to have a significant capacity increase for
charge/discharge cycles beyond the number for conventional anodes. The anodes had a capacity of more than 260 mAh/g after 700 cycles.[2]
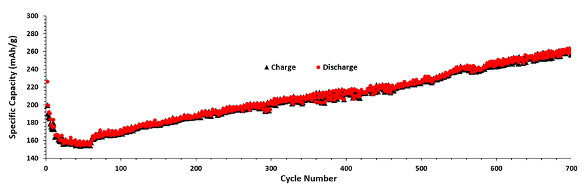
The charge/discharge plot of Portobello mushroom anodes. (Portion of Fig. 6 of ref. 2, licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License.)[2)]
The six million
electric vehicles forecast to be built by 2020 would require nearly 900,000 tons of graphite for battery anodes.Clearly, a "green" manufacturing process is required.[3] The UC Riverside research team is developing
prototype pouch batteries based on this novel type of anode.
Patents have been filed.[3]
References:
- William Shakespeare, "A Midsummer Night's Dream," via Project Gutenberg.
- Brennan Campbell, Robert Ionescu, Zachary Favors, Cengiz S. Ozkan, and Mihrimah Ozkan, "Bio-Derived, Binderless, Hierarchically Porous Carbon Anodes for Li-ion Batteries," Scientific Reports, vol. 5, article no. 14575 (September 29, 2015), doi:10.1038/srep14575, This is an open access paper with a PDF file available here.
- Sean Nealon, "Making Batteries with Portabella Mushrooms," University of California, Riverside, Press Release, September 29, 2015.
Permanent Link to this article
Linked Keywords: Materials science; materials scientists; technology; material; nature; research; superconductivity; laboratory; liquid helium; economics; economical; liquefaction of gases; liquefaction plant; gasket; leather; flexibility; cryogenics; low temperature; integrated circuit; semiconductor device fabrication; manufacturer; apricot; abrasive; deflashing; epoxy; plastic; IC package; walnut shell; mechanical properties; toxicity; non-toxic; mineral dust; atom; cubic crystal; Turkey; list of countries by apricot production; metric ton; United States; US; United States Department of Agriculture; Wikimedia Commons; Morus; mulberries; natural product; aphrodisiac; Shakespeare; A Midsummer Night's Dream; mushroom; colleague; corporate; scientist; joke; mushroom management; University of California, Riverside; Portobello mushroom; anode; lithium-ion battery; mycology; pileus; Lisa Redfern; nomenclature; Google Search; website; written language; vowel; vote; Gnumeric; carbon; chemical synthesis; synthetic; graphite; pyrolysis; sucrose; porosity; surface area; activated carbon; potassium hydroxide; battery capacity; ampere-hour; mA-h; gram; base; environmentally friendly; biomass; engineer; absorption; water; air; concentration; potassium; salt; battery; desiccation; dehydrate; pyrolysis; hierarchy; hierarchical; nanoribbon; oxygen; organic compound; potassium; chemical compound; nanometer; ransmission electron microscopy; transmission electron micrograph; Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License; ion; charge cycle; postgraduate education; graduate studen; Materials Science and Engineering; co-author; scientific literature; paper; research; mobile phone; cell phone; charge/discharge cycle; electric vehicle; forecast; prototype; patent; William Shakespeare, "A Midsummer Night's Dream."