Photonic Pigments
April 11, 2014
Colors make
foods more appealing, as demonstrated by such comestibles as
red velvet cake and
jelly beans. The association is so strong that
children will conflate
flavors and their colors. As a child, I personally enjoyed red.

Jelly bean candy is associated with Easter, but a "jelly bean" component in the electronics industry is one that's generic, inexpensive, and available from many sources.
(Photo by Brandi Sims, via Wikimedia Commons.)
Although
color additives were often used in foods, the
US Food and Drug Administration (FDA) wasn't given jurisdiction over color additives until 1960, and that was only in response to reported
toxic effects caused by some
manufacturers using too much
orange dye. In the
1970s, high doses of the popular food dye,
FD&C Red No. 2, a.k.a., Red Dye No. 2, (
amaranth, C
20H
11N
2Na
3O
10S
3) were shown to produce
cancer in
female rats, and the dye was withdrawn from use.
At the time FD&C Red No. 2 was discontinued, about a million
pounds of the dye was used
annually. Seeing Red Dye No. 2 on an ingredient label is one thing, but a
consumer confronted with its
chemical name,
trisodium (4E)-3-oxo-4-[(4-sulfonato-1-naphthyl)hydrazono]naphthalene-2,7-disulfonate, might think twice about eating it. The dye was replaced by
FD&C Red 40, C
18H
14N
2Na
2O
8S
2, lesser known as
disodium 6-hydroxy-5-((2-methoxy-5-methyl-4-sulfophenyl)azo)-2-naphthalenesulfonate. Somehow, that doesn't sound that much better.
You don't always need a dye to produce a color. The
wave nature of light allows creation of color by
diffraction and
interference effects. A film of
oil on the surface of
water functions as a
Fabry–Pérot etalon to produce color. I noticed as a child, as have many of my readers, the colorful sheen on the surface of some cuts of
luncheon meats. This arises from the periodic
reflection, and subsequent interference, of light from
striated rows of muscle cells.
This same process of color generation, called
iridescence, occurs also in the nano-textured surface of
butterfly wings, as shown in the photograph. The spiral groves on
CDs and
DVDs are spaced so closely that they will spread
white light into its
spectrum of colors like a
diffraction grating. There are
instructions on YouTube for creating a
spectrograph using a DVD and a
webcam.[1]

Dorsal view of a male Morpho didius butterfly.
The wing color is caused by nanoscale texture.
(Photo by Didier Descouens, via Wikimedia Commons.)
The problem with iridescent color is that it changes with viewing
angle. This allows for some nice
artistic effects, but iridescent materials cannot be used as a replacement for a single color dye. Now, an international team of
scientists from
Harvard University's School of Engineering and Applied Sciences, the
Korea Advanced Institute of Science and Technology (KAIST, Daejeon, Korea), and the Korea Electronics Technology Institute (Gyeonggi-do, Korea) has reported on a way to produce a palette of individual colors using "
photonic pigments."[2-3] The
research team, led by Harvard's
Vinothan N. Manoharan, reported on its experiments in a recent issue of
Angewandte Chemie International Edition.[2]
The photonic pigments are simply
microcapsules containing a
dense amorphous packing of core–shell
colloidal particles.[2] These microcapsules are assembled using
microfluidics, and they have colors that span the
visible spectrum (see figure).[2]
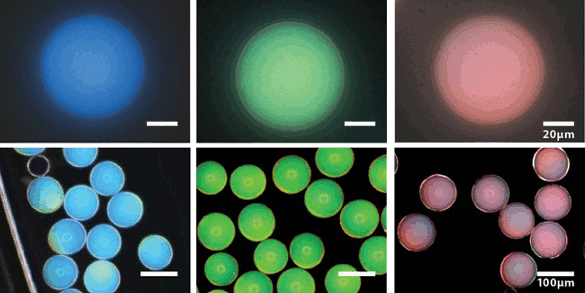
Examples of blue, green, and red photonic pigments. These images are by bright-field optical microscopy (top) and dark-field optical microscopy (bottom). (Harvard University image by Jin-Gyu Park.)[3)]
This approach to photonic pigmentation began when one of the Harvard coauthors,
Jin-Gyu Park, was at
Yale University. In his research there, he found that he could create a blue color from aggregates of solid particles.[3] In the Harvard process, microcapsules are filled with a disordered solution of even smaller particles suspended in water. As the microcapsules dry out, the microcapsules shrink, bringing the particles closer together. The interior particles aren't ordered, but there's still an average separation between the particles sets the final color, as shown in the figure.[3]
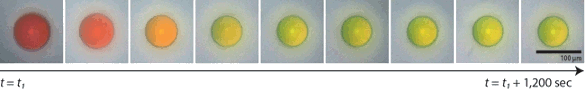
The color of a photonic pigment microcapsule shifts to shorter wavelengths as it shrinks to its final size. (Harvard University image by Jin-Gyu Park.)[3)]
Aside from the advantage of being able to produce color from non-toxic materials, the colors produced have the advantage of permanence. Dyes will eventually fade through exposure to light, but the photonic pigments achieve their colors by structure, so their colors are essentially ageless.[3] The
spherical particles could be used for sunlight-readable
electronic ink displays.[3]
This research was supported by the
National Science Foundation, both as a project grant and through support of
Harvard's Center for Nanoscale Systems. A
provisional patent application has been filed on this color capsule technology.[3]
References:
- Public Laboratory: Build a $10 USB visible-light spectrometer, YouTube video, Nov 29, 2011. Further information is available at spectralworkbench.org.
- Jin-Gyu Park, Shin-Hyun Kim, Sofia Magkiriadou, Tae Min Choi,Young-Seok Kim and Vinothan N. Manoharan, "Full-Spectrum Photonic Pigments with Non-iridescent Structural Colors through Colloidal Assembly," Angewandte Chemie International Edition, vol. 53, no. 11 (March 10, 2014), pp. 2899-2903.
- Manny Morone, "Brighter inks, without pigment," Harvard University School of Engineering and Applied Sciences Press Release, March 14, 2014.
Permanent Link to this article
Linked Keywords: Colors; food; red velvet cake; jelly bean; child; children; flavor; Easter; electronic component; Brandi Sims; Wikimedia Commons; food coloring; color additive; US Food and Drug Administration; toxicity; toxic; manufacturing; manufacturer; FD&C Orange Number 1; orange dye; 1970s; FD&C Red No. 2; amaranth; cancer; female; rat; pound; annual; consumer; chemica; Allura Red; FD&C Red 40; electromagnetic radiation; wave nature of light; diffraction; wave propagation; interference; motor oil; water; Fabry–Pérot etalon; cold cut; luncheon meat; reflection; striated muscle tissue; muscle cell; iridescence; butterfly; wing; compact disc; CD; DVD; white light; spectrum; diffraction grating; YouTube; spectrograph; webcam; Morpho didius; nanoscale; Morpho didius; Wikimedia Commons; angle; art; artistic; scientist; Harvard School of Engineering and Applied Sciences; KAIST; Korea Advanced Institute of Science and Technology; photonic crystal; pigment; research; Vinothan N. Manoharan; Angewandte Chemie International Edition; micro-encapsulation; microcapsule; density; amorphous; packing; colloid; microfluidics; visible spectrum; >bright-field optical microscopy; dark-field optical microscopy; Jin-Gyu Park; Yale University; wavelength; sphere; spherical; electronic paper; electronic ink display; National Science Foundation; Harvard Center for Nanoscale Systems; provisional patent application.