Liquid Nanoparticles
October 24, 2014
As anyone who has tried to pour
sand or
granulated sugar through a
funnel,
granular materials will often
jam so that their flow is impeded.
Non-viscous liquids will flow through a funnel with no problem, but liquids could be considered to be granular, too, although their particles are
molecules. It seems that there should be a particle size between molecules and grains of sand at which particles act more like a liquid than a
solid. However, the particles, themselves, should still behave as solids, shouldn't they?
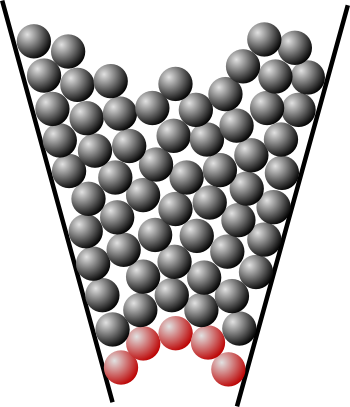
Two-dimensional sphere model of granular jamming. The jamming in this case is from formation of an arch, shown by the red spheres.
Various factors affect whether jamming will occur, but one factor ignored in this model is a distribution of particle sizes. Small spheres, mixed with the larger spheres, would enhance flow.
(Via Wikimedia Commons.)
An international team of
scientists from
Southeast University (Nanjing, China), the
Massachusetts Institute of Technology (Cambridge, Massachusetts),
Kyoto University (Kyoto, Japan),
Zhejiang University (Hangzhou, China), and the
University of Pittsburgh (Pittsburgh, Pennsylvania), led by MIT's
Ju Li, has used
in situ high-resolution transmission electron microscopy to observe
silver nanoparticles undergoing
deformation. They find that these nanoparticles can be deformed like liquid
droplets while still maintaining their interior
crystallinity. The deformation is
elastic, with no sign of
dislocation formation.[1,2]
As nanomaterials are incorporated into greater numbers of
consumer products, it's important to understand their
properties. Nanomaterials have a large
ratio of surface area to volume, so they are useful as
electrode materials in
batteries, and for
catalysts. Surface area effects can figure, also, in their use as
electrical interconnects. When a
designer specifies a shape, he presumes that that shape will persist for many years at
room temperature. If
noble metal electrical connections deform, connection will degrade or cease.[2]
The present
experiments show that ten
nanometer silver nanoparticles appear to be wobbling and changing shape, as if they were liquid droplets. Moreover, detailed
analysis shows that the particle interiors are stable and maintain the crystallinity of bulk silver metal.[1-2] The experiments were done at room temperature, far below silver's
melting point of 962
°C (1763
°F). MIT's Li says that the result would apply to
metals other than silver.[2]
A representation of atoms comprising silver nanoparticles.
(MIT image by Yan Liang.)[2)]
In the experiments, silver nanoparticles were deformed inside a transmission electron microscope so their internal crystallinity and external structure could be examined. All the liquid-like action happens in the outer one- or two-atomic layers. Atoms on these layers move along the
surface to redeposit elsewhere, but the inner core of atoms remains rigidly locked into their
lattice structure. All atoms of a liquid drop, including interior atoms, would move. Says Li,
"The interior is crystalline, so the only mobile atoms are the first one or two monolayers... Everywhere except the first two layers is crystalline.”[2]
Signs of this same effect have been seen for
tin, a metal with a much lower melting point (232 ° C) than silver.[MIT] The particle behavior is
pseudoelastic, since the particles return to their former shape when force is removed. The "pseudo-" prefix is added to indicate that the atoms have moved, but the reconstructed shape is unchanged. If the shape were changed, it would be
plastic deformation.
A similar
phenomenon, known as “
Coble creep,” was proposed for a similar sort of plasticity by MIT
ceramist,
Robert L. Coble. The silver nanoparticle behavior is an example of "Coble pseudoelasticity."[1] This effect can be eliminated by an atomically-thick
oxide layer.
Mechanically, a small
grain size promotes
fracture toughness, but after a limit, materials get weaker. Says Li, "The transition from ‘smaller is stronger’ to ‘smaller is much weaker’ can be very sharp." This crossover occurs at ten nanometers.[2]
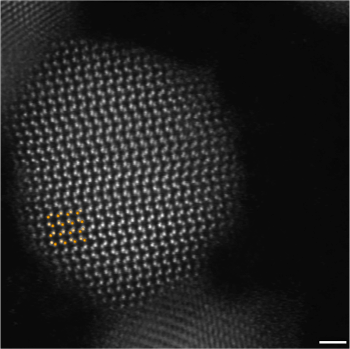
Atomic resolution image of a cadmium selenide (CdSe) nanoparticle. The whiter dots are the cadmium atoms, which reflect more electrons since they have a higher atomic number (48) compared with selenium (34).
(Electron micrograph by Robert Hovden, via Wikimedia Commons.)[3)]
![]()
References:
- Jun Sun, Longbing He, Yu-Chieh Lo, Tao Xu, Hengchang Bi, Litao Sun, Ze Zhang, Scott X. Mao, and Ju Li, "Liquid-like pseudoelasticity of sub-10-nm crystalline silver particles," Nature Materials (Advance Online Publication, October 12, 2014), doi:10.1038/nmat4105.
- David L. Chandler, "Solid nanoparticles can deform like a liquid," MIT Press Release, October 12, 2014.
- Haitao Zhang, Bo Hu, Liangfeng Sun, Robert Hovden, Frank W. Wise, David A. Muller, and Richard D. Robinson, "Surfactant Ligand Removal and Rational Fabrication of Inorganically Connected Quantum Dots," Nano Letters, vol. 11, no. 12 (October 19, 2011), pp 5356-5361.
Permanent Link to this article
Linked Keywords: Sand; sucrose; granulated sugar; funnel; granular material; jamming; viscosity; non-viscous; liquid; molecule; solid; two-dimensional space; sphere; granular material; arch; distribution; Wikimedia Commons; scientist; Southeast University (Nanjing, China); Massachusetts Institute of Technology (Cambridge, Massachusetts); Kyoto University (Kyoto, Japan); Zhejiang University (Hangzhou, China); University of Pittsburgh (Pittsburgh, Pennsylvania); Ju Li; high-resolution transmission electron microscopy; silver; nanoparticle; deformation; droplet; crystal; crystallinity; elasticity; elastic; dislocation; final good; consumer product; property; ratio of surface area to volume; electrode; material; battery; catalysis; catalyst; electrical connection; electrical interconnect; industrial design; designer; room temperature; noble metal; experiment; nanometer; analysis; melting point; celsius; °C; Fahrenheit; °F; metal; atom; Yan Liang; surface; crystal structure; lattice structure; tin; pseudoelasticity; pseudoelastic; plasticity; plastic deformation; phenomenon; Coble creep; ceramic; ceramist; Robert L. Coble; oxide; mechanics; crystallite; grain; fracture toughness; atom; atomic resolution; cadmium selenide; cadmium; electron; atomic number; selenium; electron microscope; electron micrograph.