Diamond Thread
October 15, 2014
The expression, "
tough as nails," is flawed in two respects. First, the word, "tough," in this expression is used to connote
hardness or
yield strength, while
materials scientists reserve
toughness to express
fracture strength. The
science of fracture toughness, which I wrote about in a
previous article (Atmospheric Dust, February 2, 2011), was pioneered by
Alan Arnold Griffith. In 1921, Griffith proposed a
fracture law that relates the
ultimate strength of a
brittle solid to the size of its largest flaw.
Second, while the strength of the
steel from which
nails are made is considerable, steel is not a strong as some
materials.
Young's modulus, which is a measure of how a material will
deform when
stress is applied, is about 200
GPa for steel.
Diamond has a Young's modulus of 1,220 GPa, but stronger still is another
allotrope of carbon,
carbyne (linear acetylenic carbon), that is predicted to have a Young's modulus as high as 32,000 GPa. I wrote about carbyne in a
previous article (Carbyne, August 28, 2013).
Intermediate in scale between carbyne and diamond is another carbon allotrope,
fullerene, exemplified by the sixty-
carbon atom, ball-shaped
molecule,
buckminsterfullerene, C
60, as shown in the figure. Although this molecule in its natural state is soft, like
graphite, aggregates of buckminsterfullerene, called
fullerite, become harder than diamond when
compressed. Considerable research on this material has been conducted by Mikhail Popov, V. Blank, and their
colleagues at various Institutions in
Moscow, Russia.[1-3], most recently, this year.[4-5] Fullerite has an
isothermalbulk modulus of 491 GPa, as compared with about 445 GPa for diamond.
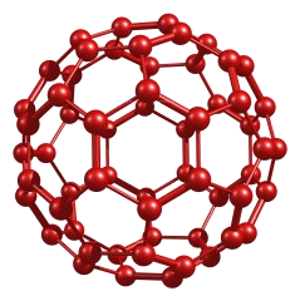
Diagram of buckminsterfullerene, C60.
(Moscow Institute of Physics and Technology State University image.)[5)]
Like the old
detective movie cliché of proving that a supposed diamond is real by scratching
mirror glass with it, the
Moh's hardness scale is based on which
minerals will scratch each other. Diamond is at the top of this scale, having a Moh's hardness of 10, since it will scratch every other mineral. Although some minerals lower on the Moh's scale will produce small, visible marks on diamond, these marks are quite distinguishable from real scratches. That's why it's significant that the
diamond anvils used in fullerite synthesis by the Moscow team were damaged, as shown in the photograph.
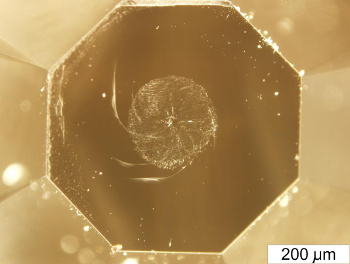
Diamond anvil damaged during synthesis of ultrahard fullerite.
(Moscow Institute of Physics and Technology State University image by Mikhail Popov.)[5)]
In the current Russian process, fullerene is subjected to a mechanical
pressure of 6-6 GPa in the presence of
carbon disulfide (CS
2).[4] This produces the same material that previously required 18 GPa; and, in each case, the diamond anvils that provided the pressure were damaged. It was also possible to produce fullerite at 13 GPa at an elevated
temperature of about 820
°C (1100
K).[5] The
bulk modulus of the diamond anvils was 585 GPa, and
analytical techniques showed that
sulfur is not incorporated into the fullerite material.[4]
The resulting fullerite is a
polymer of carbon in which the fullerene molecules are
bonded together to form a
three-dimensional polymer. [5] Says Mikhail Popov, head of the Laboratory of Functional Nanomaterials at the Technological Institute for Superhard and Novel Carbon Materials, and an author of the study,
“The discovery described in this article (the catalytic synthesis of ultrahard fullerite) will create a new research area in materials science because it substantially reduces the pressure required for synthesis and allows for manufacturing the material and its derivatives on an industrial scale."[5]
Instead of making bulk fullerite, scientists from
Pennsylvania State University (University Park, Pennsylvania), the
Carnegie Institution of Washington (Washington DC), and
Arizona State University (Tempe, Arizona), have instead made diamond-like
threads of carbon polymer.[6-8] These nanothreads are long, thin strands of carbon atoms having the same bonding arrangement as the diamond structure in which each carbon atom is
tetrahedrally-coordinated with the others. High pressure applied to
benzene forms
cyclohexane rings of six carbon atoms bonded together, and these rings are bonded to each other (see figure). The edge bonds are terminated by
hydrogen.[7]
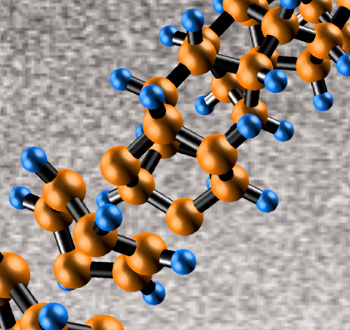
Artist's impression of the structure of a diamond nanothread.
It's a chain of cyclohexane molecules with hydrogen atoms shown in blue.
(Penn State University image by Enshi Xu.)
Says
John Badding, a professor in the
Penn State Chemistry Department and
corresponding author for the published study,
"From a fundamental-science point of view, our discovery is intriguing because the threads we formed have a structure that has never been seen before... It is as if an incredible jeweler has strung together the smallest possible diamonds into a long miniature necklace... Because this thread is diamond at heart, we expect that it will prove to be extraordinarily stiff, extraordinarily strong, and extraordinarily useful."[7]
To create these diamond threads, the research team compressed a six
millimeter diameter column of benzene. Slow release of pressure after
room temperature compression allowed the carbon atoms to link up in a highly ordered chain of the cyclohexane molecular units.[7] During compression, the flat benzene molecules first stack together, then they bend and break apart. As the pressure is released, the carbon atoms reconnect into the diamond nano-thread structure.[7]
X-ray and
neutron diffraction,
Raman spectroscopy,
solid-state NMR,
transmission electron microscopy and
first-principles calculations confirmed the structure, which is
crystalline in two dimensions.[6] It has
short-range order in the other dimension, but portions of these diamond nanothreads are less than perfect.[6-7] It's
conjectured that the carbon atoms in the benzene molecules, after having their original bonding destroyed, bond differently as pressure is removed, so a polymerization occurs to form the diamond nanothread structure.[7]
Since the thread width is
atomically small, such threads could potentially become an important lightweight, strong, stiff material.[7] Says Badding,
"You can attach all kinds of other atoms around a core of carbon and hydrogen. The dream is to be able to add other atoms that would be incorporated into the resulting nanothread. By pressurizing whatever liquid we design, we may be able to make an enormous number of different materials... One of our wildest dreams for the nanomaterials we are developing is that they could be used to make the super-strong, lightweight cables that would make possible the construction of a space elevator, which so far has existed only as a science-fiction idea."[7]
This research was funded by the
U.S. Department of Energy.[7]
References:
- V. Blank, M. Popov, S. Buga, V. Davydov, V. N. Denisov, A. N. Ivlev, B. N. Mavrin, V. Agafonov, R. Ceolin, H. Szwarc, and A. Rassat, "Is C 60 fullerite harder than diamond?" Physics Letters A. vol. 188, no. 3 (May 23, 1994), pp. 281-286.
- V. D. Blank, S. G. Buga, N. R. Serebryanaya, G. A. Dubitsky, B. Mavrin, M. Yu. Popov, R. H. Bagramov, V. M. Prokhorov, S. A. Sulynov, B. A. Kulnitskiy and Ye. V. Tatyanin, "Structures and physical properties of superhard and ultrahard 3D polymerized fullerites created from solid C60 by high pressure high temperature treatment.," Carbon, vol. 36, nos. 5-6 (1998), pp. 665-670.
- V. Blank, M. Popov, G. Pivovarov, N. Lvova, K. Gogolinsky, and V. Reshetov, "Ultrahard and superhard phases of fullerite C60 : comparison with diamond on hardness and wear," Diamond and Related Materials, vol. 7, nos. 2-5 (February, 1998), pp. 427-431.
- M. Popov, V. Mordkovich, S. Perfilov, A. Kirichenko, B. Kulnitskiy, I. Perezhogin, and V. Blank, "Synthesis of ultrahard fullerite with a catalytic 3D polymerization reaction of C60," Carbon, vol. 76 (September, 2014, doi: 10.1016/j.carbon.2014.04.075), pp. 250-256.
- Scientists come closer to the industrial synthesis of a material harder than diamond, Moscow Institute of Physics and Technology State University Press Release, September 15, 2014.
- Thomas C. Fitzgibbons, Malcolm Guthrie, En-shi Xu, Vincent H. Crespi, Stephen K. Davidowski, George D. Cody, Nasim Alem, and John V. Badding, "Benzene-derived carbon nanothreads," Nature Materials, Advance Online Publication (September 21, 2014, doi:10.1038/nmat4088).
- Scientists use 'smallest possible diamonds' to form ultra-thin nanothreads, Penn State University Press Release, September 22, 2014.
- John Badding, professor of chemistry at Penn State, talks about his research, YouTube Video by Penn State University, September 21, 2014.
Permanent Link to this article
Linked Keywords: Tough as nails; hardness; yield strength; materials science; materials scientist; toughness; fracture strength; fracture mechanics; Alan Arnold Griffith; Griffith's criterion; strength of materials; ultimate strength; brittleness; brittle; steel; nail; material; Young's modulus; plasticity; deform; stress; pascal; GPa; Diamond; allotrope of carbon; linear acetylenic carbon; carbyne; fullerene; carbon atom; molecule; buckminsterfullerene; graphite; fullerite; compression; compressed; colleague; Moscow, Russia; isothermal; bulk modulus; Moscow Institute of Physics and Technology State University; film noir; detective movie; cliché; mirror; glass; Mohs scale of mineral hardness; Moh's hardness scale; mineral; diamond anvil cell; Mikhail Popov; pressure; carbon disulfide; temperature; Celsius; °C; kelvin; bulk modulus; scientific instrument; analytical technique; sulfur; polymer; chemical bond; three-dimensional space; catalysis; catalytic; chemical synthesis; industrial scale; Pennsylvania State University (University Park, Pennsylvania); Carnegie Institution of Washington (Washington DC); Arizona State University (Tempe, Arizona); thread; tetrahedral symmetry; tetrahedrally-coordinated; benzene; cyclohexane ring; hydrogen; artist; Enshi Xu; John Badding; Penn State Chemistry Department; academic authorship; corresponding author; jeweler; necklace; millimeter; diameter; cylinder; column; room temperature; X-ray crystallography; neutron diffraction; Raman spectroscopy; solid-state nuclear magnetic resonance; NMR; transmission electron microscopy; ab initio; first-principles; calculation; crystal; crystalline; short-range order; conjecture; atom; atomic; space elevator; science-fiction; U.S. Department of Energy.