Batteries from Sand
July 16, 2014
Early
scientific research was focused on simple
materials, since those were the only ones available.
Materials scientists learned a lot about
iron before they tackled iron
alloys. Now, many
centuries hence, all the
low-hanging fruit has been
harvested, and scientists can only do something really novel if they have a novel material. Fortunately, some of these materials, like
graphene, are just different forms of an old standard.
However, most novel materials are novel because they contain exotic, and usually
toxic or otherwise dangerous,
chemical elements. For example,
thin film photovoltaic materials contain
cadmium,
selenium and
tellurium. Advanced
semiconductor devices, such as
diode lasers, contain
arsenic.
Lithium batteries and
lithium-ion batteries contain
reactive compounds of
lithium.

No, it's not a poorly hung painting by Piet Mondrian. It's a blank "NFPA 704" label for material hazard identification.
The blue, red and yellow areas would contain number codes, respectively, for health hazard, flammability, and chemical reactivity. The white area is for special hazard information.
(Via Wikimedia Commons.)
Many scientists have started to think "
green" by attempting to replace existing materials and the
processes for their
manufacture with
environmentally friendly and
renewable alternatives. I wrote about the idea of replacing some
structural materials with
cellulose fiber composites in a
recent article (Strong Cellulose Filaments, July 2, 2014).
In a recent
publication, materials scientists and
engineers from the
University of California - Riverside (UCR) have described their process for the production of
nanoscale silicon anodes for
lithium ion batteries. Their process incorporates some "green"
chemistry by using
sand (
quartz silica crystals) and table salt (
sodium chloride, NaCl) as starting materials. Their anodes are better than the conventional
graphite material, producing batteries with a
capacity of 1,024
milliamp-
hours per
gram when
discharged at 2 amps per gram after 1000 cycles.[1-2]
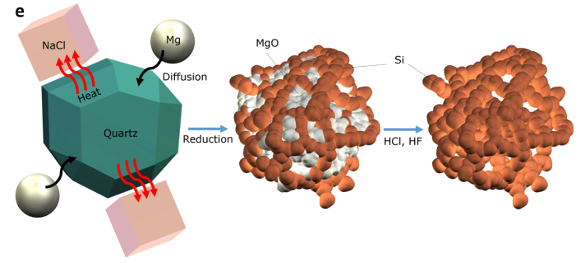
Using magnesium and sodium chloride to turn sand into nanosilicon. (University of California, Riverside, image.)[2)]
Their process, as shown in the above figure, involves the reduction of silica (SiO
2) by
magnesium to produce
magnesium oxide and silicon; viz.,
SiO2 + 2Mg -> Si + 2MgO
One feature of the process is the use of NaCl as a
heat absorption medium. The highly
exothermic reaction of magnesium with quartz would proceed violently were the NaCl not present.[2] The heat scavenging NaCl also promotes the formation of nano-silicon
network with interconnect thickness of 8-10
nm (see figure).[1
One non-green aspect of the process is that an
acid mixture of
hydrochloric and
hydrofluoric acid is required to remove the magnesium oxide.[1] Also, the harvested sand needs to be
milled to
nanometer scale and then
purified.[2] Examples of the starting materials and final nanosilicon product are shown in the photographs, below.[2]
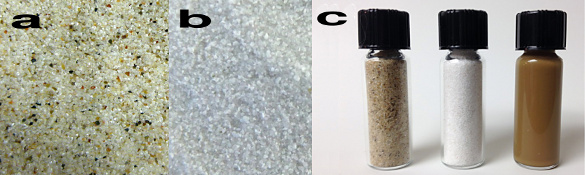
From start to finish: (a) unpurified sand, (b) purified sand, and (c) vials of unpurified sand, purified sand, and nano silicon. (University of California, Riverside, image. modified for clarity.)[2)]
This research was prompted by the fact that the optimization limit has been reached for graphite, the present anode material for lithium-ion batteries. Silicon, being
conductive like graphite, is an alternative, but it's difficult to produce nanoscale silicon in large quantity. The
porosity of the nanoscale silicon produced by the UCR process is what makes this anode material ideal for its purpose.[2]
Batteries made from this nanoscale silicon could have three times the useful
lifespan of batteries used for
electric vehicles, and they would power
mobile electronic devices three times longer. The research team is scaling their research from
coin-sized batteries to larger sizes.[2] The University of California, Riverside, has filed
patent applications on this
invention.[2]
References:
- Zachary Favors, Wei Wang, Hamed Hosseini Bay, Zafer Mutlu, Kazi Ahmed, Chueh Liu, Mihrimah Ozkan and Cengiz S. Ozkan, "Scalable Synthesis of Nano-Silicon from Beach Sand for Long Cycle Life Li-ion Batteries," Scientific Reports, vol. 4, Article no. 5623 (July 8, 2014), DOI:10.1038/srep05623. This is an open access article with a PDF file available here.
- Sean Nealon, "Using Sand to Improve Battery Performance," University of California at Riverside Press Release, July 8, 2014.
Permanent Link to this article
Linked Keywords: Science; scientific; research; material; materials scientist; iron; alloy; century; low-hanging fruit; harvest; graphene; toxicity; toxic; chemical element; thin film solar cell; thin film photovoltaic material; cadmium; selenium; tellurium; semiconductor device; laser diode; diode laser; arsenic; lithium battery; lithium-ion battery; chemical reaction; reactive; chemical compound; lithium; Piet Mondrian; NFPA 704; hazard; toxicity; health hazard; flammability; chemical reactivity; Wikimedia Commons; green; industrial process; manufacturing; manufacture; environmentally friendly; renewable resource; structural material; cellulose; fiber; composite; scientific literature; publication; engineer; University of California, Riverside; nanoscopic scale; nanoscale; silicon; anode; lithium ion battery; chemistry; sand; quartz; silicon dioxide; silica; crystal; sodium chloride; graphite; battery capacity; milliamp; hour; gram; discharging; discharged; magnesium; sodium chloride; magnesium; magnesium oxide; enthalpy of fusion; heat absorption medium; exothermic reaction; random graph; network; nanometer; nm; acid; hydrochloric acid; hydrofluoric acid; milled; purified; electrical conductor; conductive; porosity; longevity; lifespan; electric vehicle; mobile electronic device; button cell; coin-sized battery; patent application; invention.