Green Plants, Pink House
August 5, 2013
The
Earth was described as a "
pale blue dot" by
astrophysicist,
Carl Sagan. The dot was a
sub-pixel image of the Earth taken at a distance of 3.7 billion
miles (6 billion
kilometers) by the
Voyager 1 spacecraft in 1990. The blue color came mostly from
Rayleigh scattering from
clouds in the
atmosphere.
A blue dot might be how
extraterrestrials would first image the Earth. However, for most people here on
Earth's surface we live on a
green planet because of all the
vegetation.
There's a simple reason why vegetation is green. It's a consequence of the specific
chemicals,
chlorophyll a and
chlorophyll b, which
plants use to harvest
solar energy. The green
wavelengths of
light are not useful for
photosynthesis, so they are not
absorbed by plants, and they are instead
reflected (see figure). This may be why
human vision evolved to be especially sensitive to green light. If you see it, it might be
dinner.
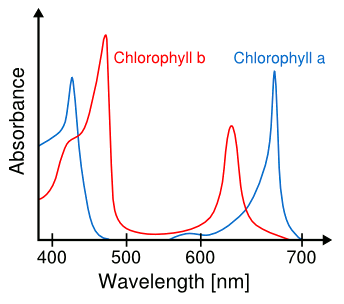
Spectra of chlorophyll a and chlorophyll b.
Green light, which is nominally 550 nm, is not absorbed.
(Modified image from Wikimedia Commons.)
Colonists on
Mars, for example, would be wise to heed the evidence of
science when it comes to the efficient growth of
food. Mars is significantly less
insolated than the Earth, as its lower
temperatures show. This is a simple consequence of the
inverse-square law for the distance from the Sun. For this reason, Martian
greenhouses need to be both
thermally insulated and supplied with
artificial light. As the
spectra in the above figure indicate, red-rich and blue-rich lighting would be most
efficient.
Scientists at
Purdue University, the
Kennedy Space Center, and
Orbital Technologies Corporation have just published results of their experiments on
tomato (Solanum lycopersicum) culture using red and blue
light-emitting diodes (LEDs).[1-3] Light-emitting diodes are far more
efficient than other light sources, such as
high-pressure sodium lamps (HPS) and typical overhead lighting (OHL) as used for room illumination. Tomato would be a good choice for Martian greenhouse food, since it will grow onto vertical
wires, making efficient use of space.
This study showed that the
electrical conversion efficiency of LED illumination into
fruit biomass was 75% higher than HPS and OHL light sources.[1] There are quite a few factors that would encourage such a growth method here on Earth, as well as on Mars. The principal factor is the high cost and large
carbon footprint of the traditional
horticultural system of growing such fruits in warmer
climates and then
transporting them thousands of miles to where they're eaten.[2] The
United States imports about a third of its tomatoes, which are picked green to
ripen during shipment, a process that reduces fruit quality.[2]
The LEDs run cool, so they can be placed in close proximity to the plants. They can illuminate not just the tops of leaves, but the bottoms, also. Says study coauthor Gómez,
"The leaves are photosynthesizing on the lower parts of the plants, and that may be helping with the plant's energy... We're getting the high intensity of the LEDs close to the plants because they're not hot like a high-pressure sodium lamp. If you put one of those close to the plants, you'd scorch it."[2]

Purdue University agricultural scientists, Cary Mitchell (left), and Celina Gómez, tend to tomatoes grown using red and blue light-emitting diodes.
(Purdue Agricultural Communication photo by Tom Campbell.)
Quite a few others are developing this ultimate "
locally-grown"
technology for horticulture, including designs that integrate living spaces with their own food production environments.[3] Although the Purdue experiments used LEDs to supplement
natural light, some others are working on garden factories that are completely enclosed and out of reach of
insects and
extreme weather.[3]
Caliber Biotherapeutics has a 150,000
square-foot "pink house" facility in
Texas in which more than two million plants are grown in vertical stacks up 50
feet high.[3] Instead of growing tomatoes, this facility grows a high-value-added
tobacco-like plant for
drug and
vaccine production.[3] One advantage of the enclosed system is that
water use is minimal, which is something that may become an
important future factor.
Let's switch topics from future photosynthesis to photosynthesis that occurred more than
two billion years ago. The origin of
oxygenic photosynthesis, the
splitting of water molecules into
oxygen upon which
animal life on Earth depends, is unknown. As in other
evolutionary processes, it's unlikely that the process we see today was the first process that
nature tried.
A team of
geobiologists from the
California Institute of Technology (Pasadena, California), the
Stanford Synchrotron Radiation Lightsource (Stanford University, Menlo Park, California), the
Massachusetts Institute of Technology (Cambridge, Massachusetts), and the
Tokyo Institute of Technology (Tokyo, Japan) have just published evidence in the
Proceedings of the National Academy of Sciences that photosynthesis evolved from a transitional photosystem involving single-
electron oxidation reactions of
manganese.[4-5]
As
materials scientists and
inorganic chemists know, manganese is a slippery character, having a myriad of oxidation states, including +2, +3, +4, +6 and +7. As such, it's easy for it to be a part of
photoreactions. As summarized by study coauthor
Woodward Fischer,
assistant professor of
geobiology at Caltech,
"Water-oxidizing or water-splitting photosynthesis was invented by cyanobacteria approximately 2.4 billion years ago and then borrowed by other groups of organisms thereafter... Algae borrowed this photosynthetic system from cyanobacteria, and plants are just a group of algae that took photosynthesis on land, so we think with this finding we're looking at the inception of the molecular machinery that would give rise to oxygen."[5]
The research team based its
hypothesis on the analysis of manganese deposits in
drill core specimens of 2.415 billion-year-old
South African marine
sedimentary rocks.[4-5] The analysis showed that the manganese was deposited as the
oxide, and not as manganese that was subsequently oxidized as oxygen on the Earth increased.[4-5]

Caltech graduate student Jena Johnson, a coauthor of the PNAS paper, examines a South African rock formation where evidence of a 2.415 billion year old manganese-oxidizing photosystem was found.
(Caltech photo.)[5)]
The research team also plans to analyze manganese-bearing rocks from western
Australia similar in age to the South African samples.[5] However, the most interesting part of the research program is the plan to
mutate cyanobacteria to perform manganese-oxidizing photosynthesis. Investigation of such a system, says Fischer, "could help target technologies for energy production from artificial photosynthesis."[5]
References:
- Celina Gómez, Robert C. Morrow, C. Michael Bourget, Gioia D. Massa and Cary A. Mitchell, "Comparison of Intracanopy Light-emitting Diode Towers and Overhead High-pressure Sodium Lamps for Supplemental Lighting of Greenhouse-grown Tomatoes," HortTechnology, vol. 23, no. 1 (February, 2013 ), pp. 93-98.
- Brian Wallheimer, "LEDs reduce costs for greenhouse tomato growers, study shows," Purdue University Press Release, April 29, 2013.
- Michaeleen Doucleff, "Vertical 'Pinkhouses:' The Future Of Urban Farming?" NPR, May 21, 2013.
- Jena E. Johnson, Samuel M. Webb, Katherine Thomas, Shuhei Ono, Joseph L. Kirschvink and Woodward W. Fischer, "Manganese-oxidizing photosynthesis before the rise of cyanobacteria," Proc. Natl. Acad. Sci., Online before Print, June 24, 2013, doi: 10.1073/pnas.1305530110.
- Katie Neith, "A Stepping-Stone for Oxygen on Earth," Caltech Press Release, June 26, 2013.
Permanent Link to this article
Linked Keywords: Earth; pale blue dot; astrophysics; astrophysicist; Carl Sagan; pixel; mile; kilometer; Voyager 1 spacecraft; Rayleigh scattering; cloud; atmosphere of Earth; atmosphere; extraterrestrial; lithosphere; Earth's surface; green; planet; vegetation; chemical; chlorophyll a; chlorophyll b; plant; solar energy; wavelength; light; photosynthesis; absorption of electromagnetic radiation; reflection; visual perception; human vision; dinner; green light; nanometer; nm; Wikimedia Commons; settler; colonist; Mars; science; food; insolation; temperature; inverse-square law; greenhouse; thermal insulation; thermally insulated; artificial light; spectrum; spectra; energy conversion efficiency; efficient; scientists; Purdue University; Kennedy Space Center; Orbital Technologies Corporation; tomato; light-emitting diode; high-pressure sodium lamp; wire; electricity; electrical; fruit; biomass; carbon footprint; horticultural system; climate; transport; United States; ripening; Cary Mitchell; Tom Campbell; local food; locally-grown; technology; sunlight; natural light; insect; extreme weather; Caliber Biotherapeutics; square-foot; Texas; foot; feet; Nicotiana; tobacco-like; drug; vaccine; water; water security; geologic time scale; oxygen; oxygenic; water splitting; splitting of water molecules; animal life; evolution; evolutionary process; nature; geobiology; geobiologist; California Institute of Technology (Pasadena, California); Stanford Synchrotron Radiation Lightsource (Stanford University, Menlo Park, California); Massachusetts Institute of Technology (Cambridge, Massachusetts); Tokyo Institute of Technology (Tokyo, Japan); Proceedings of the National Academy of Sciences of the United States of America; electron; oxidation reaction; manganese; materials science; materials scientist; inorganic chemistry; inorganic chemist; photocatalysis; photoreaction; Woodward Fischer; assistant professor; geobiology; cyanobacteria; algae; hypothesis; drill core specimen; South Africa; sedimentary rock; oxide; Australia; mutation; mutate.