Ocean Manganese
September 20, 2013
When I was in
graduate school in the early
1970s, there was a lot of
press about
mining sea floor manganese nodules. These nodules are so-named because they are mostly
manganese (27-30%), although they contain quite a number of other useful metals, such as
nickel (1.25-1.5%),
copper (1-1.4%) and
cobalt (0.2-0.25%). They are sometimes called polymetallic nodules, since they incorporate these and other
metals as well. I wrote about these nodules in a
previous article (The Manganese Conspiracy, June 9, 2010).

Sea floor manganese nodules.
(United States Geological Survey photograph, via Wikimedia Commons.)
These
baseball-sized nodules are produced over the course of
tens of millions of years by slow
geological processes that include
precipitation of the metal
hydroxides by
microorganisms. In any case, the
chemistry is simple. If the concentration of a dissolved metal exceeds its
solubility, it will leave solution, generally attaching itself to a suitable substrate.
Manganese and nickel are useful
elemental additions to
alloys. Manganese, which is often used in the formulation of
stainless steels, will capture dissolved
oxygen and
sulfur from the melt and render a better solid. Manganese is used also as an alloying element for
aluminium. Manganese at about 1.5% gives aluminum
corrosion resistance, and this particular alloy composition is used in aluminum
beverage cans. Nickel is used in
superalloys.
The base price of manganese is not that high, presently about a
dollar per
pound. Nickel, however, is an expensive metal, presently about $6.50/pound, with considerable price fluctuation, as shown in the figure. In the 1970s, when the price of nickel was just $1-$2/pound, there was talk about harvesting manganese nodules from the sea floor, not just for the manganese, but the nickel as well.
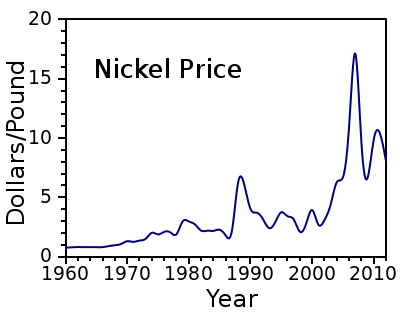
The price of nickel, 1960-2012, from US Geological Survey data.[1-2]
(Plot by author, using Gnumeric.)
To prevent the usual "
tragedy of the commons" scenario with everyone rushing to harvest these valuable nodules from the sea, the
United Nations published its
Convention on the Law of the Sea in 1982; and it established an
International Seabed Authority in 1994. There are presently
regulations for nodule harvesting with protection of the marine
environment. The
United States is not a signatory of the Law of the Sea Convention. Since nodule harvesting is technically challenging enough without governmental interference, most companies do not presently envision nodule harvesting as being
economically viable.
The early
hyperbole of manganese nodule economics was actually a cover story for a US
Central Intelligence Agency operation. What the public thought was an investigation of nodule harvesting by reclusive billionaire industrialist,
Howard Hughes, was actually a front for
Project Azorian, an attempt to recover
K-129, a
Soviet submarine that sank in deep water in April, 1968.
The
salvage vessel, the
Hughes Glomar Explorer, was launched in 1974 with the purported purpose of harvesting manganese nodules from the ocean floor. As the story goes (with the caveat that it's usually not wise to believe everything a government tells you), only a third of the K-129 submarine was recovered. This third did not contain such coveted items as
nuclear missiles and
code book, but it did contain some
cryptographic equipment.

The Hughes Glomar Explorer
(Via Wikimedia Commons.)
There's still some
basic research being undertaken on oceanic manganese, as a recent paper in
Science proves.[3-4] Scientists from the
University of Delaware (
Lewes, Delaware), the
Oregon Health and Science University (
Beaverton, Oregon),
McGill University (
Montreal, Quebec) and the
Université du Québec à Rimouski (
Rimouski, Québec), looked at the
oxidation states of manganese in sea water. Manganese is an essential nutrient in
photosynthesis. Says paper coauthor,
George Luther of the University of Delaware,
"You wouldn't think manganese is that important, but without manganese, we wouldn't have the molecular oxygen that we breathe."[4]
Manganese has several oxidation states, and the research team found that oceanic manganese(III) is far more prevalent than previously thought.[4] In previous studies of this element, only the total dissolved manganese was measured, and it was presumed that it was all manganese(II).[3-4]
The first indication that Mn(III) might be prevalent was a study by Luther in the mid-2000s in which this oxidation state was found in
Black Sea waters in which a
gradient in
oxygen concentration is present. In such water, oxygen levels are relatively high near the surface, and they diminish with depth.[4]
Studies showed that Mn(III) was also present in the sea floor mud, not only at the Black Sea, but also at a
Gulf of Saint Lawrence and a Delaware
salt marsh having the same oxygen gradient.[4] Soluble Mn(III) is likely stabilized by
organic or
inorganic ligands, and the research team thinks that it accounts for up to 90% of dissolved manganese.[3] It's produced by oxidation of dissolved Mn(II) and by
reductive dissolution of MnO
2 by biological or non-biological processes.[3]
As I wrote earlier, precipitation of metal hydroxides by microorganisms is a process in the formation of manganese nodules, and the published study offers more insight into this mechanism. Says study coauthor,
Andrew Madison, who did this research as part of his graduate studies at the University of Delaware,
"In sediments, bacteria prefer to consume molecular oxygen and nitrate first due to their high energy gain... After those are consumed, bacteria then couple organic matter oxidation to manganese oxide reduction, which can produce soluble manganese(III)."[3]
This research was funded by the
National Science Foundation and the
Natural Sciences and Engineering Research Council of Canada.[3]
References:
- Average annual nickel prices through 1990, US Geological Survey
- Average annual nickel prices, 1991-2012, US Geological Survey.
- Andrew S. Madison, Bradley M. Tebo, Alfonso Mucci Bjorn Sundby and George W. Luther III, "Abundant Porewater Mn(III) Is a Major Component of the Sedimentary Redox System," Science, vol. 341, no. 6148 (August 23, 2013), pp. 875-878.
- Teresa Messmore, "Morphing manganese - UD researchers report new discovery in 'Science' about manganese in aquatic environments," University of Delaware Press Release, August 22, 2013.
Permanent Link to this article
Linked Keywords: Graduate school; graduate school; 1970s; mass media; press; mining; seabed; sea floor; manganese nodule; manganese; nickel; copper; cobalt; metal; United States Geological Survey; Wikimedia Commons; baseball; geologic time scale; tens of millions of years; geology; geological; precipitation; hydroxide; microorganism; chemistry; solubility; chemical element; elemental; alloy; stainless steel; oxygen; sulfur; aluminium; corrosion resistance; beverage can; superalloy; dollar; pound; Gnumeric; tragedy of the commons; United Nations; Convention on the Law of the Sea; International Seabed Authority; regulation; environment; United States; economics; economically; hyperbole; Central Intelligence Agency; Howard Hughes; Project Azorian; Soviet submarine K-129; Soviet; submarine; marine salvage; Hughes Glomar Explorer; nuclear missile; code book; cryptography; cryptographic; pure research; basic research; Science journal; University of Delaware; Lewes, Delaware; Oregon Health and Science University; Beaverton, Oregon; McGill University; Montreal, Quebec; Université du Québec à Rimouski; Rimouski, Québec; oxidation state; photosynthesis; George Luther; Black Sea; gradient; oxygen; Gulf of Saint Lawrence; salt marsh; organic ligand; inorganic ligand; redox; reduction; Andrew Madison; nitrate; energy; National Science Foundation; Natural Sciences and Engineering Research Council.