Icicle Ripples
November 11, 2013
I'm a native of
Upstate New York, which can get quite cold and snowy in the
winter months. My
undergraduate and
graduate education was in upstate locales, and one winter season at
Syracuse University, we were subjected to more than 140
inches of
snowfall. Surprisingly, this amount is not that far from the
mean. My wife still recalls the time when one of the
students was rendered immobile after stepping off the
campus bus into a snow bank. Two
football players exited the bus to pull her out.
The
scientifically-minded undergraduates would relax in those winter months by building devices to make artificial
icicles. Addition of
food coloring to the
water gave some very
artistic renderings, which, like
performance art, would run its course, and disappear in the
spring. As you can imagine, some of these icicles were huge.

Unsafe at any speed.
The property of ice with which most people are familiar is its slipperiness. This is caused by a thin layer of water on its surface. The origin of this water has been debated for more than a century with no proper resolution.
(FEMA image by Michael Raphael, via Wikimedia Commons.)
Ice was even a topic of our
seminars. I can remember a
materials scientist, naturally from
Canada, who gave a talk about his
research on the
extrusion of
ice. Just as the purpose of
medical research on
animals is usually not to cure the animals, the research on ice extrusion was not because extruded ice is useful. It was just a way to use the
transparency of ice to make extrusion research easier to do.
Once again the Canadians have stepped forward to push back the frontiers of ice research, this time in an investigation of why some icicles have ripples on their surface.
Physicists from the
University of Toronto have found that icicles grown from
pure water do not exhibit surface ripples and the addition of
non-ionic surfactants does not produce surface ripples. However, even small
ion concentrations of
ionic impurities dissolved in water will induce ripples, and the growth rate of ripples increases very weakly with ionic concentration.[1-3]
The Toronto study was done by
experimental physicist,
Stephen Morris, and Antony Szu-Han Chen, a
Ph.D. candidate. [2] One
theory of ripple formation in icicles is that they are caused by the modification of
surface tension as the result of the water film that flows along the surface during growth. This theory was directly tested by the introduction of surfactants.[2] Says Antony Chen, who was lead author of the research paper on this study, "Nobody has systematically investigated what causes the ripples, so we began growing them in the lab."
They grew 67 icicles, varying the
factors that might have an influence, such as
ambient temperature,
flow rate of water, the motion of the air surrounding it, and the
composition of the source water.[2-3] Their
growth apparatus, placed in a
refrigerated box,
rotated the growing icicles so they would grow evenly on all sides. Their outlines were
digitally imaged, and
computer analyzed to reveal the icicle
cross-section.[2]
The clue to the importance of water purity came from the observation that
Toronto tap water produced ripply icicles, while
distilled water didn't. The tap water is reasonably pure, but the threshold for rippling is only 20
milligrams of
salt per
liter.[2] The growth rate of the ripples followed a very weak, roughly
logarithmic dependence on
ion concentration.[2-3] Analysis of icicles harvested from
nature confirmed the rippling dependence on salt concentration.[2]
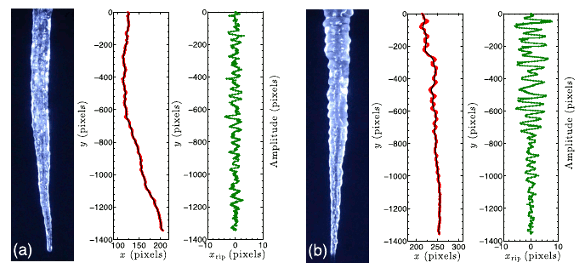
A picture is worth a thousand words. Left images (a) are for distilled water, and right images (b) are for distilled water with the addition of 0.008 wt-% NaCl. Red data are the detected edge, and green data are the ripple positions for the left edges. (Images by Antony Szu-Han Chen and Stephen W. Morris from fig. 2 of ref. 1, licensed under a Creative Commons License.)
One interesting feature of icicle growth is that the rippling has a characteristic
wavelength, no matter the size of the icicle or where it grows.[3] Aside from the surface tension effect in growth, which these experiments discredited, there's another
theory involving
heat transfer. Water is presumed to
freeze faster at the peaks of the ripples, since
heat is removed most effectively there.[3] This results in a
positive feedback mechanism in which a ripple promotes more growth at the ripple.
Theory needs to catch up with experiment. Says Stephen Morris,
"Our results have provided strong empirical evidence, but as of yet we don't have a theoretical explanation as to why the impurities have this effect. Neither do we have a theory for why the ripples have a universal wavelength – this still remains a central mystery."[3]
Although much research, such as this, is driven by
curiosity, there might also be some important applications. Ice from freezing rain can cause serious problems for
power lines,
ships,
bridges and
airplanes.[3] This research was funded by the
Natural Sciences and Engineering Research Council of Canada.[3]
References:
- Antony Szu-Han Chen and Stephen W Morris, "On the origin and evolution of icicle ripples," New Journal of Physics, vol. 15, no. 10 (October, 2013), article 103012.
- Want ripples on your icicles? University of Toronto scientists suggest adding salt, University of Toronto Press Release, October 10, 2013.
- Water impurities key to an icicle's ripples, Institute of Physics Press Release, October 10, 2013.
Permanent
Link to this article
Linked Keywords: Upstate New York; winter; undergraduate education; undergraduate; graduate school; graduate education; Syracuse University; inch; snowfall; mean; student; campus; bus; football player; science; scientifically-minded; icicle; food coloring; water; art; artistic rendering; performance art; spring; ice; slipperiness; Wikimedia Commons; seminar; materials scientist; Canada; research; extrusion; ice; medical research; animal; transparency; physicist; University of Toronto; purified water; pure water; ion; non-ionic; ionic; surfactant; concentration; experimental physicist; Stephen Morris; Doctor of Philosophy; Ph.D.; theory; surface tension; factor; ambient temperature; flow rate; composition; laboratory equipment; growth apparatus; refrigerator; refrigerated box; rotation; rotate; digital camera; digitally imaged; image analysis; cross-section; Toronto; tap water; distilled water; milligram; salt; liter; logarithm; logarithmic; nature; A picture is worth a thousand words; svodium chloride; NaCl; Creative Commons License; wavelength; theory; heat transfer; freezing; freeze; heat; positive feedback; curiosity; power line; ship; bridge; airplane; Natural Sciences and Engineering Research Council of Canada.