Graphene Micro-Supercapacitors
February 27, 2013
Supercapacitors have become useful
energy storage devices. I've reviewed some aspects of supercapacitors in two previous articles (
Flow Batteries, July 18, 2012 and
Carbon Nano-Onion Supercaps, August 26, 2010). It's always worthwhile reviewing the
equations describing the simplest
capacitor, the
parallel plate capacitor.
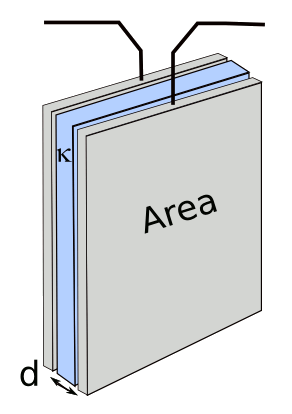
C = κε
oA/d
Q = C V
I = ∂Q/∂t
W = (1/2) C V
2
V = V
o e
(-t/RC)
Q = C V
o e
(-t/RC)
I = (V
o/R) e
(-t/RC)
where C is
capacitance, κ is the dielectric constant, εo is the permittivity of free space, A is the area of the capacitor plates, d is the plate separation, Q is the charge, V is the voltage, Vo is the initial voltage, I is the current, W is the stored energy, t is time, and R is the load resistance.
Since my brain is tuned for picofarads (10-12 F), I use 8.854 x 10-12 farads per
meter for ε
o. If you use farads as the units of capacitance, the stored energy will have units of
joules. A
watt is a joule per second.
You can see from these equations that large plate area, small plate separation and high dielectric constant lead to high capacitance; and, thereby, high energy storage at high voltage. In
electric double-layer supercapacitors, the dielectric layer is the interface between
conductive electrolyte layers.
This very thin dielectric interface, combined with the use of very high area
carbon electrodes made from
activated charcoal, gives capacitors of many farads in a few
cubic inches.
Electrochemical decomposition of the electrolyte, however, limits operation to about 3 - 5 volts.
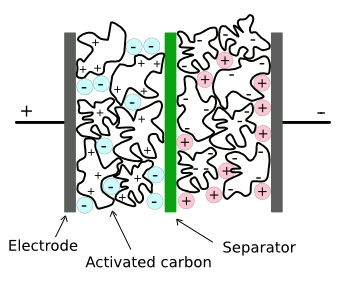
Structure of a conventional supercapacitor.
There are separate ionic liquids that are negatively and positively charged. One disadvantage of current materials is that these ionic liquids decompose at voltages above a few volts.(Via Wikimedia Commons, modified))
So, how does the
energy density of supercapacitors compare with other energy stores? As the table shows, there's still considerable room for improvement. The 0.07 kJ/gram estimate is for commercial supercapacitors at this time.
Many
university laboratories have been doing research on improving supercapacitor energy storage density. Since
graphene is the miracle
material of the moment, it's no surprise that some of this
research is about using graphene in supercapacitors. A considerable advance in the
state of the art has been achieved by
scientists from the
UCLA NanoSystems Institute, and they've published the results of their study in a recent issue of
Nature Communications.[1-3]
Richard Kaner, a
professor of
Chemistry and Biochemistry,
Inorganic Chemistry, and
Materials Science and Engineering, and
research associate,
Maher F. El-Kady, have developed a process for fabrication of graphene micro-supercapacitors.
In the same tradition as
Ernest Rutherford's doing experiments with string and sealing wax, they fabricated these with a
LightScribe DVD burner.[1-2] The
laser imaging in the
DVD burner is quite precise, allowing a precise definition of device features.[2] This deviation from traditional
photofabrication is explained by Kaner, as follows:
"Traditional methods for the fabrication of micro-supercapacitors involve labor-intensive lithographic techniques that have proven difficult for building cost-effective devices, thus limiting their commercial application... Instead, we used a consumer-grade LightScribe DVD burner to produce graphene micro-supercapacitors over large areas at a fraction of the cost of traditional devices. Using this technique, we have been able to produce more than 100 micro-supercapacitors on a single disc in less than 30 minutes, using inexpensive materials."[2]
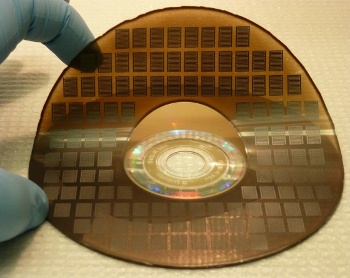
UCLA micro-supercapacitors, as produced by Kaner and El-Kady's DVD burner process
(UCLA Photograph.)[2)]
In the UCLA process, a layer of
plastic is glued to a DVD, and the plastic is then coated with a
graphite oxide dispersion in
water.[2] The graphite oxide is transformed by the laser light into graphene.[2] The capacitors are formed as interdigitated electrodes of graphene topped with an
ionogel electrolyte. Close electrode spacing reduces the
diffusion path for the electrolyte ions, so the micro-supercapacitors can charge at a higher rate than other capacitors.[2]
Measurements demonstrate a power density of about 200 W-cm
-3, which puts these at the top rank of supercapacitor energy density.[1] The micro-supercapacitors also show excellent cycling stability which is much better than micro-batteries; and they can be charged a hundred to a thousand times faster than batteries.[2] They also have the advantage that they are highly bendable and twistable.[2]
References:
- Maher F. El-Kady and Richard B. Kaner, "Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage," Nature Communications, vol. 4 (February 12, 2013), Article no. 1475, doi:10.1038/ncomms2446.
- Davin Malasarn, "UCLA researchers develop new technique to scale up production of graphene micro-supercapacitors," University of California. Los Angeles, Press Release, February 19, 2013.
- Brian Golden Davis, Director, "The Super Supercapacitor," Vimeo video.
Permanent Link to this article
Linked Keywords: Electric double-layer capacitor; supercapacitor; energy storage; equation; capacitor; parallel plate capacitor; capacitance; relative permittivity; dielectric constant; vacuum permittivity; permittivity of free space; area; electric charge; voltage; electric current; energy; time; electrical resistance; farad; meter; joule; watt; electric double-layer supercapacitor; electrical conductor; conductive; electrolyte; carbon; electrode; activated charcoal; cubic inch; electrochemical; decomposition; Wikimedia Commons; energy density; svupercapacitor; Lead Acid Battery; NiMH Battery; Flywheel; Lithium Battery; Ethanol; Gasoline; Hydrogen; university; laboratory; graphene; material; research; state of the art; scientist; University of California, Los Angeles; UCLA; NanoSystems Institute; Nature Communications; Richard Kaner; professor; Chemistry and Biochemistry; Inorganic Chemistry; Materials Science and Engineering; research associate; Maher F. El-Kady; Ernest Rutherford; LightScribe DVD burner; laser; DVD; photolithography; photofabrication; plastic; graphite oxide; dispersion; water; ion gel; ionogel; diffusion.