Graphene Gas Barrier Composite
October 30, 2013
Many
experiments are conducted under
vacuum, and
scientists have been aided by the many advances in vacuum
technology since 1643, when the
Italian physicist,
Evangelista Torricelli, first produced a
laboratory vacuum by inverting a closed container filled with
mercury. The historical
unit of
pressure, the
torr, symbolizing a
millimeter's height of mercury, is named after him. The torr is presently defined as 1/760 of a
standard atmosphere.

Evangelista Torricelli (1608-1647)
When Torricelli inverted a mercury column in 1643 to form the first technical vacuum, he incidentally created the first barometer.
The asteroid, 7437 Torricelli, is named in honor of Torricelli's achievements.
(Via Wikimedia Commons.)
As any
experimentalist who's worked with a vacuum can attest, most of your time is spent waiting for your system to
pump to a desired vacuum level and looking for
leaks. Most experiments operate under
ultrahigh vacuum condition, which means a pressure less than about 10
-9 torr (about 10
-7 pascals). In my vacuum experiments, I was lucky in needing a vacuum level of just 10
-6 torr, but I was just as unlucky in having leaks.
The
poorly-funded experimentalist looking for vacuum leaks in
my generation used a technique that is not allowed by today's
safety standards. To find a leak, you would spray
acetone around each
joint, looking for a tell-tale jump in pressure. Today, most laboratories use
helium leak detection in which a simple
mass spectrometer, such as a
residual gas analyzer, detects a
helium spray near a leaky joint.
Helium is an ideal material for leak testing, since helium
atoms are small and
neutral. Helium will easily flow through even small leaks, so helium gas is used as a means to test the
hermeticity of
electronic component packaging.[1] Helium will
permeate (flow through) most materials, finding a path through voids between atoms, so helium permeation is a useful test of
packaging materials.
Drug packages, such as
blister packs, need to protect the drugs not only from exposure to the
gases in
air (
oxygen is the usual problem), but also
moisture. Some
materials are better at preventing oxygen permeation, and others will prevent
water permeation, so most barrier packaging has layers of more than one material. It took a while to replace
glass as a container for
carbonated beverages for the simple reason that the
carbon dioxide molecules would leak through most
plastics. The worldwide
market for rigid barrier materials is nearly $100 billion annually.[2]
Graphene is the miracle material of the past decade, so its utility is being explored for many applications. It's intrinsic
strength, and its structure as a thin, tightly
bonded assemblage of
carbon atoms has been used to advantage by scientists at
Rice University and their
colleagues at the
University of Szeged,
Hungary, the
University of Ljubljana,
Slovenia and the
Cochin University of Science and Technology,
India, to produce a
composite material of graphene and
polyurethane that acts as a very effective barrier material. Their research is reported in an advanced online edition of
ACS Nano.[3]
The
research team mixed 0.5 wt.-%
hexadecyl-functionalized low-defect
graphene nanoribbons (HD-GNRs) in
thermoplastic polyurethane (TPU).[3] Thin films of the composite were created by
solution-casting.[4] The
nitrogen gas permeability was reduced by three
orders of magnitude by this small addition of graphene, which also increased the strength of the composite.[3] When used as a barrier between a nitrogen atmosphere and a vacuum, films without the addition of the HD-GNRs caused a complete loss of vacuum in a hundred seconds, while the films with the HD-GNRs showed no change in vacuum after a thousand seconds, and just a small change after 18 hours.[4]
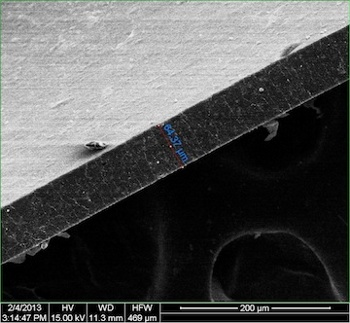
An electron micrograph of a 65 micrometer thick composite film of graphene nanoribbons in polyurethane, viewed edge-on.
Such films were prepared by solution-casting.
(Rice University image by Changsheng Xiang.)[4)]
The gas barrier arises from an overlap of the finely
dispersed 200-300
nanometer-wide ribbons which act nearly as a single sheet of graphene encompassing the entire material. The gas molecules can't penetrate the graphene, so they need to maneuver a tortuous path through the thickness of the material.[4] Presently, graphene can't be produced in the large quantities needed for the routine production of barrier films, but
James M. Tour of Rice University previously published a technique to "unzip"
multiwalled carbon nanotubes into graphene nanoribbons.[5-6]

An electron micrograph of graphene nanoribbons embedded in a block copolymer.
(Rice University image by Changsheng Xiang.)[4)]
A potential application of this material might be as
storage tanks for
natural gas vehicles, where the combination of light weight and strength is desirable. Says Tour,
"The idea is to increase the toughness of the tank and make it impermeable to gas... This becomes increasingly important as automakers think about powering cars with natural gas. Metal tanks that can handle natural gas under pressure are often much heavier than the automakers would like."[4]
This research was funded by the
Air Force Research Laboratory and the
Office of Naval Research.[4]
References:
- Standard ASTM E493/E493M - 11, "Standard Practice for Leaks Using the Mass Spectrometer Leak Detector in the Inside-Out Testing Mode," ASTM International.
- Rick Lingle, "Barrier materials for rigid packaging nears $95 billion for 2012," Packaging Digest, February 11, 2013.
- Changsheng Xiang, Paris J Cox, Akos Kukovecz, Bostjan Genorio, Daniel P Hashim, Zheng Yan, Zhiwei Peng, Chih-Chau Hwang, Gedeng Ruan, Errol L. G. Samuel, Parambath M Sudeep, Zoltan Konya, Robert Vajtai, Pulickel M Ajayan and James M. Tour, "Functionalized Low Defect Graphene Nanoribbons and Polyurethane Composite Film for Improved Gas Barrier and Mechanical Performances," ACS Nano Just Accepted Manuscript (DOI: 10.1021/nn404843n, October 8, 2013).
- Mike Williams, "Tanks, graphene! Rice advances compressed gas storage," Rice University Press Release, October 10, 2013.
- Dmitry V. Kosynkin, Amanda L. Higginbotham, Alexander Sinitskii, Jay R. Lomeda, Ayrat Dimiev, B. Katherine Price and James M. Tour, "Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons," Nature, vol. 458, no. 7240 (April 16, 2009), pp. 872-876.
- Geoff Brumfiel, "Nanotubes cut to ribbons - New techniques open up carbon tubes to create ribbons," Nature News, April 15, 2009.
Permanent Link to this article
Linked Keywords: Experiment; vacuum; scientist; technology; Italian; physicist; Evangelista Torricelli; laboratory; mercury; unit; pressure; torr; millimeter; standard atmospheric pressure; standard atmosphere; Evangelista Torricelli (1608-1647); barometer; 7437 Torricelli; Wikimedia Commons; experimentalist; vacuum pump; leak; ultrahigh vacuum; pascal; science policy; poorly-funded; baby boomer; Occupational Safety and Health Administration; OSHA; safety standard; acetone; pipe joint; helium mass spectrometer; helium leak detection; mass spectrometry; mass spectrometer; residual gas analyzer; RGA; helium; atom; neutral; hermetic seal; hermeticity; electronic component; integrated circuit packaging; permeation; permeate; packaging material; prescription drug; blister pack; gas; atmosphere of Earth; air; oxygen; moisture; material; water; glass; carbonated beverage; carbon dioxide; molecule; plastic; market; graphene; strength of materials; strength; chemical bond; bonded; carbon; Rice University; collegiality; colleague; University of Szeged; Hungary; University of Ljubljana; Slovenia; Cochin University of Science and Technology; India; composite material; polyurethane; ACS Nano; research; hexadecane; hexadecyl-functionalized; graphene nanoribbon; thermoplastic; solution-casting; nitrogen gas; orders of magnitude; electron microscope; electron micrograph; micrometer; Changsheng Xiang; dispersion; dispersed; nanometer; James M. Tour; multiwalled carbon nanotube; block copolymer; storage tank; natural gas vehicle; Air Force Research Laboratory; Office of Naval Research; Standard ASTM E493/E493M - 11, "Standard Practice for Leaks Using the Mass Spectrometer Leak Detector in the Inside-Out Testing Mode," ASTM International.