Evaporation
August 7, 2013
One bit of
physical chemistry that most
children learn at a very young age is that
liquids evaporate more quickly on hot days than cold days. That observation might come from noticing that a splash of
beverage spilled on a
shirt dries quickly in the
summer; or, seeing how fast a
sidewalk dries after a summer's
rain. The really observant child, one destined to become a
scientist, might even notice that the evaporation rate depends on
wind speed.
Thermodynamically, both the
thermal and wind-driven processes are easily understood. The wind drives away
vapor molecules that linger just above the liquid, so it makes the air less
saturated with the liquid molecules.
Equilibrium at the liquid-air interface means that the same number of molecules leave the liquid to become vapor as those condensing from the vapor to form more liquid. The wind shifts the equilibrium towards more evaporation.
The temperature effect is quantified by the
Clausius–Clapeyron equation, which relates how the
vapor pressure of a liquid changes with temperature from an initial state (1) to a final state (2); viz.,
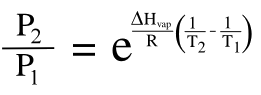
where ΔHvap is the enthalpy of vaporization, R is the gas constant (8.314 J K−1 mol−1), P is the pressure and T is the absolute temperature. The exponential term means that even a modest temperature change will cause a large change in vapor pressure. The vapor pressure of water (ΔHvap = 40.68 kj/mol) at 25 °C will be 30% larger than that at 5 °C.
Scientists from the
Institute of Physical Chemistry of the Polish Academy of Sciences (
Warsaw, Poland) have just completed a study of evaporation of liquid
droplets over a wide range of droplet sizes. Their results are published in the journal,
Soft Matter.[1-2] In good scientific form, some of their
experiments had droplets evaporating, not in
air, but in an
inert gas.

Research at the Institute of Physical Chemistry, the Polish Academy of Sciences, shows that temperature differences as small as 0.0001 kelvin drive droplet evaporation.
(IPC/PAN photograph by Grzegorz Krzyzewski.)[2)]
The Clausius-Clapeyron equation is derived from
equilibrium thermodynamics where it's assumed that molecules can attach or detach from the fluid with ease to enter the vapor phase. It's also assumed that the liquid and the vapor are at the same temperature, although
evaporation will cool water. The overall principle is that the evaporation happens slowly enough that the temperature equilibrates.
The Polish group attacked the problem of droplet evaporation using a
computer model and validating
experiments. The computer model was a
molecular dynamics simulation of a two-component fluid in which molecular interactions were via a
Lennard-Jones potential. The purpose of the simulation was to calculate the
energy flux between the liquid droplets and the surrounding atmosphere.[1] Droplet size extended down to
nanoscale dimension.[2]
In the experiments, microdroplets were held in an
electrodynamic trap where they were studied as they evaporated. This was like a
Millikan oil drop experiment in which the drops evaporated. Studied systems were water evaporating in air,
glycol and
glycerol into
nitrogen, and
argon into argon vapor.[2]
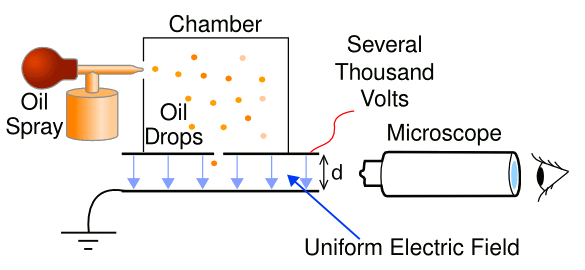
Simplified schematic diagram of the Millikan oil drop experiment. The voltage is adjusted to keep a droplet floating in the region between the plates. (Modified illustration from Wikimedia Commons.)
There's a boundary layer between the liquid and vapor that's
thermally insulating, and this layer limits the heat flux. The thickness of this boundary layer was found to depend primarily on the conditions in the
environment and it's not related to the droplet size. For this reason, nanometer-sized droplets evaporate more slowly than expected, essentially because the interface has few molecules, and the heat flux is by
radiation, and not by
mass transfer.[2]
What was found in the experiments was a clear difference in temperature between droplets and the surrounding atmosphere, and that the pressure remained constant during evaporation. The droplet temperature appears to be the prime factor in droplet evaporation.[2] Says Daniel Jakubczyk, a coauthor of the paper,
"Evaporation turns out to be a process driven by very small temperature differences. Often, only ten-thousandth parts of kelvin are enough to make it happen!"[2]
The experimental conditions of different liquids and varied droplet sizes over three
orders of magnitude resulted in evaporation times from seconds to tens of minutes.[1] The model equation derived from these observations gives good results over this wide range of conditions; that is, it appears to specify a universal law.[1] As Marek Litniewski, another study coauthor, summarized,
"Small droplets can evaporate within nanoseconds, whereas large drops need up to a few tens of minutes. The experiments confirmed that in spite of such a large time span, extending over a dozen of orders of magnitude, our formula correctly describes the kinetics of all these processes."[2]
This research can be applied to some currently important areas, such as the contribution of water evaporation to
climate change. Water vapor is Earth's primary
greenhouse gas. It may also lead to the design of better
fuel injectors to increase
internal combustion engine efficiency.[2] This work was funded by the
Polish Ministry of Science and Higher Education.[2]
As for drops of a different
genre, "
Drops of Jupiter (Tell Me)" is a popular song by the American musical group,
Train. It was released in 2001, but it still has frequent
airplay, today.[3]
References:
- Robert Holyst, Marek Litniewski, Daniel Jakubczyk, Marcin Zientara and Mariusz Wozniak , "Nanoscale transport of energy and mass flux during evaporation of liquid droplets into inert gas: computer simulations and experiments," Soft Matter, Advance Article, June 11, 2013, DOI: 10.1039/C3SM50997D.
- Milikelvins drive droplet evaporation, Institute of Physical Chemistry of the Polish Academy of Sciences Press Release, July 18, 2013 (PDF File).
- Train, "Drops Of Jupiter," YouTube Video. This video has 33 million views!
Permanent Link to this article
Linked Keywords: Physical chemistry; child; children; liquid; evaporation; evaporate; drink; beverage; shirt; summer; sidewalk; rain; scientist; wind speed; thermodynamics; thermodynamically; temperature; thermal; vapor; molecule; saturation; saturated; equilibrium; Clausius–Clapeyron equation; vapor pressure; enthalpy of vaporization; gas constant; joule; J; kelvin; K; mole; mol; pressure; absolute temperature; exponentiation; exponential; water; celsius; °C; Institute of Physical Chemistry of the Polish Academy of Sciences; Warsaw, Poland; drop; droplet; Soft Matter; experiment; atmosphere of Earth; air; inert gas; Grzegorz Krzyzewski; equilibrium thermodynamics; evaporative cooling; computer model; experiment; molecular dynamics; Lennard-Jones potential; energy flux; nanoscopic scale; nanoscale dimension; classical electromagnetism; electrodynamic; Millikan oil drop experiment; glycol; glycerol; nitrogen; argon; schematic diagram; voltage; Wikimedia Commons; thermal insulation; environment; thermal radiation; mass transfer; order of magnitude; climate change; greenhouse gas; fuel injector; internal combustion engine; energy conversion efficiency; Polish Ministry of Science and Higher Education; genre; Drops of Jupiter (Tell Me); Train; airplay.