125 Years of Aluminum
October 16, 2013
Materials are so important to
culture that
archaeologists have named some periods of
human activity by their premier materials. Thus, in the
three-age system, we've lived in the
Stone Age, which ended about 4000
BC when
metalworking was practiced; the
Bronze age, which started around 2500 BC; and the
Iron Age, which started around 1000 BC.
Since
gold is the least
reactive metal, it occurs in nature as the metal, itself, so it was the first metal used by man. After gold, primitive man discovered ways to release other metals from their
ores by
heating. The
ancients were able to
smelt silver,
mercury,
copper,
lead,
tin, and finally,
iron.
Bronze, an alloy of copper and tin, was a stronger metal than copper, and it had sufficient properties to launch the Bronze Age.

Hesiod, Works and Days, lines 150-151, scanned from the author's copy of ref. 1. The translation is "Their armor was of bronze, and their houses of bronze, and of bronze were their implements: there was no black iron."[1)]
Iron smelting, the extraction of iron from its common ores, is much more difficult than the extraction of the other metals. The
reduction of the common
iron ore,
hematite (
Fe2O3 using
carbon as a
reducing agent is straightforward; vis.,
2Fe2O3 + 3C -> 4Fe + 3CO2
This so-called
carbothermic reaction uses readily available materials, but it happens at higher temperatures than encountered for the smelting of the other metals known to the ancients.
Iron is a
strong material, but iron
rusts, so our
ancestor metallurgists were always on the lookout for better metals. One of these is
aluminum, which is known as aluminium outside the US. Although
iron oxide is somewhat hard to reduce to pure iron,
aluminum oxide is much, much harder to reduce. The following table illustrates this fact through the
free energies of formation (ΔG
f) of these two compounds at some representative
temperatures.[2]
| | Oxide | ΔGf, 1000 K
kcal/mole | ΔGf, 1500 K
kcal/mole |
| | Fe2O3 | -134.082 | -104.585 |
| | Al2O3 | -325.397 | -285.975 |
The most common aluminum ore is
bauxite, which is a mixture of
aluminum hydroxides. Since aluminum is a stronger reducing agent than
carbon, a carbothermic reaction cannot be used to produce aluminum from any of its ores.
Electrochemistry lets you drive chemical reactions too difficult for thermal processing, but typical
aqueous electrochemistry will not produce aluminum, since the aluminum will react with the water. Water, however, isn't the only
solvent, and that's the key of the
Hall–Héroult process for production of aluminum. This process was independently invented by the
Frenchman,
Paul Héroult, and the
American chemist,
Charles Martin Hall in 1886. Hall
commercialized the process in 1888 by creating the
Alcoa corporation, which celebrated its 125th anniversary on October first.[3-4]
The solvent used in the Hall–Héroult process is
cryolite (Na
3AlF
6), which needs to be heated to about 1,000 °C (1,830 °F) before it's a
liquid.
Aluminium fluoride (AlF
3) can be added to form a reduced
melting point composition (see figure).
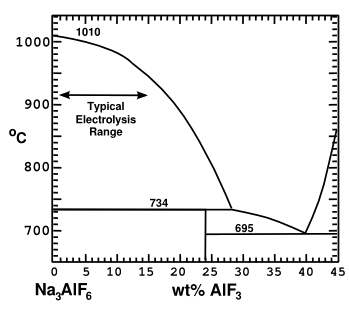
Cryolite-rich portion of the cryolite-aluminum fluoride phase diagram.
(Illustration by the author using Inkscape.)
In the Hall–Héroult process aluminum oxide is dissolved in molten cryolite and then electrolyzed with an applied
voltage of up to five volts in an
electrochemical cell with a carbon
cathode and
anode. Since the solvent temperature is above the melting point of aluminum (660°C), aluminum is deposited as a liquid at the cathode, and it sinks to the bottom of the cell.
Carbon dioxide (CO
2) is produced at the anode, which is consumed, along with some
hydrogen fluoride gas. The HF is
neutralized to make
sodium fluoride, and the CO
2 is vented to the
atmosphere; alas, as another contributor to
global warming. The process is
energy-intensive, since it takes about 13-15
kilowatt-hours of
electricity to produce a
kilogram of aluminum, which is about half the daily electrical consumption of an average
US home.
Although the Hall–Héroult process allowed a relatively inexpensive method of making aluminum, aluminum had been available for quite some time before that, having been first
isolated in 1827. Before Hall–Héroult, aluminum was prepared by a reduction of
gaseous anhydrous aluminum chloride by liquid
sodium metal at high temperature, the aluminum chloride being prepared from aluminum oxide, and the sodium prepared by electrolysis of sodium hydroxide; viz,
Al2Cl6(g) + 6Na(l) -> 2Al(s) + 6NaCl(s)
When the
Washington Monument was built, it was topped with an aluminum
apex, a solid
pyramid about a
foot high, weighing about 2.85 kg, fabricated in 1884. In 1884, aluminum was as valuable as silver, about a dollar an ounce ($25/ounce in
today's money). The intended purpose of the apex was as a
lightning rod for the monument. The chemical composition of the metal, as analyzed at the time, was 97.49-97.75% aluminum, with 1.70-1.90% iron, and 0.55-0.61%
silicon.[5]

Master Mechanic, P. H. McLaughlin, fitting the aluminum apex on the Washington Monument, 1884.
(Harper's Weekly illustration by S.H. Nealy, United States Library of Congress Prints and Photographs division digital ID cph.3b44599, via Wikimedia Commons.)
![]()
References:
- Hugh G. Evelyn-White, Trans., "Hesiod, The Homeric Hymns and Homerica," William Heinemann/The Macmillan Co., London/New York, 1914, pp. 12-13.
- L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- Len Boselovic, "Alcoa celebrates 125 years, remembers roots," Pittsburgh Post-Gazette, October 1, 2013.
- Aluminum candles: Alcoa, Pittsburgh-born and bred, marks 125 years, Pittsburgh Post-Gazette, October 3, 2013.
- George J. Binczewski, "The Point of a Monument: A History of the Aluminum Cap of the Washington Monument," JOM, vol. 47, no. 11 (1995), pp. 20-25.
Permanent Link to this article
Linked Keywords: Material; culture; archaeology; archaeologist; human; three-age system; Stone Age; Anno Domini; BC; metalworking; Bronze age; Iron Age; gold; chemical reaction; reactive; metal; ore; heat; ancients; smelting; silver; mercury; copper; lead; tin; iron; bronze; Hesiod; Works and Days; ferrous metallurgy; iron smelting; redox; reduction; iron ore; hematite; iron III oxide; Fe2O3; carbon; reducing agent; carbothermic reaction; strength of materials; strong material; rust; ancestor; metallurgy; metallurgist; aluminum; iron oxide; aluminum oxide; standard Gibbs free energy of formation; free energy of formation; temperature; calorie; kcal; mole; bauxite; aluminum hydroxide; carbon; electrochemistry; aqueous solution; solvent; Hall–Héroult process; Frenchman; Paul Héroult; American; chemist; Charles Martin Hall; commercialization; Alcoa corporation; cryolite; liquid; aluminium fluoride; melting point; phase diagram; Inkscape; voltage; electrochemical cell; cathode; anode; carbon dioxide; hydrogen fluoride; neutralization; neutralized; sodium fluoride; atmosphere of Earth; global warming; energy-intensive; kilowatt-hour; electricity; kilogram; United States; US; chemical element discovery; isolation; gas; gaseous; anhydrous; aluminum chloride; sodium metal; Washington Monument; apex; pyramid; foot; time value of money; today's money; lightning rod; silicon; Harper's Weekly; United States Library of Congress; Wikimedia Commons.