Radioactive Fountains of the Deep
March 29, 2012
There's more than just
liquids, such as
lava and
groundwater, that flow from the depths of the
Earth onto the
surface and the
atmosphere. There is, of course,
natural gas, which is on our mind because of its
energy value; and also the
helium that's contained in natural gas and is harvested for its obvious value.
Natural gas contains more than 5% helium, much more than the 0.00052% by volume that's found in the atmosphere, and it's extracted using the
fractional distillation process known and loved by all
organic chemistry undergraduates. This helium is created by the
radioactive decay of
thorium and
uranium; for example,
238U -> 234Th + 4He
232Th -> 228Ra + 4He
Since their symbols are similar, the
radium, Ra, in the decay of thorium shouldn't be confused with
radon, Rn. The most common
isotope of radon,
222Rn is produced in the alpha decay of
226Ra
226Ra -> 222Rn + 4He
Since radium-226 is a common
trace element in many
rocks, quite a bit of radon is produced and released into the environment. Since
222Rn has a
half-life of just 3.8 days, that equates to a lot of
natural radioactivity. It's estimated that 2,400 million
curies (9 x 10
13 becquerels) of radon are annually released into the environment.[1]
Humans have existed on the Earth for quite a while, so our exposure to radon can't be all that bad, or we would be
extinct. Radon was essentially ignored until 1985, when radiation alarms at the entrance of a
nuclear power plant were triggered by an employee. The interesting thing about this event was that the plant had not yet been
fueled. An investigation revealed a radon level of 100,000 Bq/m
3 in the
basement of his house. A typical indoor radon reading is about a thousand times less than this.
The modern problem of radon exposure comes from our living indoors, and not in the great outdoors. The enclosed space of our homes, now nicely sealed to
save energy, traps the radon that enters from cracks and gaps in the
foundation where our homes interface to Earth's surface. The
US Environmental Protection Agency has a web site devoted to radon mitigation in homes.[2]
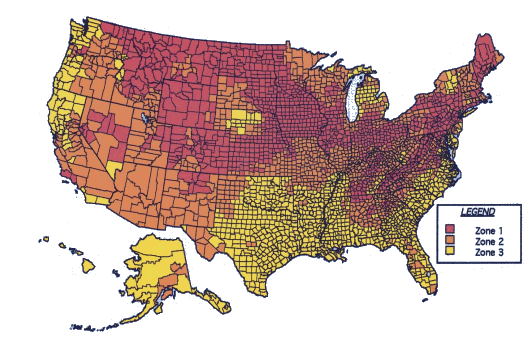
Radon map of the United States (EPA). Zone 1 > 4 pCi/L (picocuries per liter); Zone 2 between 2 and 4 pCi/L; Zone 3 <2 pCi/L. (via epa.gov web site))
If all this scares you into pitching a
tent in a
forest to escape radon, a paper published last year by
scientists from the
International Laboratory for Air Quality and Health, the
Queensland University of Technology (Brisbane, Australia), presents evidence that trees harvest radon from groundwater.[3-4] This conclusion came from a study investigating why the concentration of
ions in forest areas is consistently higher than in other areas. Natural ions in the air are created by
cosmic rays, and by the radiative decay of radon.[4]
Radon is
water-soluble, so it's taken up by
trees and
plants through the groundwater they absorb, and it's released into the atmosphere by
transpiration. The study estimates that a forest composed of
eucalyptus trees, spaced four
meters apart, would account for about 37% of atmospheric radon during the middle of the day when the rate of transpiration is highest.[3] However, when the value is annualized, the contribution drops to just four percent.[3] Said Queensland scientist,
Dr Rohan Jayaratne,
"Trees act as radon pumps, bringing the gas to the surface and releasing it to the atmosphere through transpiration - a process where water absorbed by the root system is evaporated into the atmosphere from leaves. This is especially prevalent for trees with deep root systems, such as eucalypts."[4]
The ions produced by the radon could be a problem, also. In experiments at six locations around the Brisbane area, the Queensland research team found that the
positive and
negative ion concentrations in the air in heavily wooded areas were about twice that of open, grassy areas.[Q] About half of the particles inhaled remain in the
respiratory system for a time, and
charged particles are more likely to deposit in the
lungs. This effect is harmful if there are
pollutants in the air.[4]

A tree of a different color.
An infrared image of a maple tree.
In such images, hotter regions are white, and cooler regions are black.
(Via Wikimedia Commons))
How can I write an article about trees without a mention of the mighty
larch,[5] or
lumberjacks?[6]
![]()
References:
- J.H. Harley, Environmental Radon, in Noble Gases, Richard E. Stanley and A. Alan Moghissi, Environmental Research Center (Las Vegas, 1975), pp. 109-114 (via Google Books).
- Radon - Indoor Air, US Environmental Protection Agency.
- E.R. Jayaratne, X. Ling and L. Morawska, "Role of Vegetation in Enhancing Radon Concentration and Ion Production in the Atmosphere," Environ. Sci. Technol., vol. 45, no. 15 (August 1, 2011), pp. 6350–6355.
- Electricity from trees, Queensland University of Technology Press Release, March 21, 2012.
- Monty Python, "The Larch Sketch," YouTube video.
- Monty Python, "The Lumberjack Song," YouTube video; German version; and, a very thorough review on Wikipedia.
Permanent Link to this article
Linked Keywords: Liquid; lava; groundwater; Earth; surface; atmosphere; natural gas; energy value; helium; fractional distillation; organic chemistry; undergraduate; radioactive decay; thorium; uranium; radium; radon; isotope; trace element; rock; half-life; natural radioactivity; curie; becquerel; human; extinct; nuclear power plant; fuel; basement; energy conservation; foundation; Environmental Protection Agency; tent; forest; scientist; International Laboratory for Air Quality and Health; Queensland University of Technology (Brisbane, Australia); ion; cosmic rays; solubility; water-soluble; tree; plant; transpiration; eucalyptus tree; meter; Rohan Jayaratne; positive; negative; respiratory system; charged particle; lung; pollutant; Wikimedia Commons; larch; lumberjacks; The Lumberjack Song.