Local Order in Metallic Glasses
October 17, 2012
Scientists of
my generation didn't
interview like other potential
employees. We believed that actions spoke louder than words; so, if our
publication record doesn't excite you, we shouldn't be having this conversation. This can be encapsulated in the
Latin phrase,
esse quam videre, "To be, rather than seem to be." Not surprisingly, this phrase has been used as a
motto by
many organizations, including the
Berklee College of Music.
When I interviewed for the job that would encompass my thirty year career in
industrial research, I did what most people wouldn't do. I argued with the
director of the
laboratory, a distinguished member of the
National Academy of Engineering, about a
scientific issue. He and his colleagues had been doing research in
metallic glasses. These are
metal alloys with no apparent
crystal structure; that is, the
atoms are not
ordered in space like the atoms of normal
metals.
He claimed that there was no order at all, including what's called
short-range order. I told him that's because he hadn't been looking hard enough. Shortly thereafter, quite elaborate
experiments involving
synchrotron X-ray sources showed that such order did exist.
I had been encouraged in this argument by a conversation I had in
graduate school with an aged
physical chemist. He had told me about an
X-ray experiment he had done on
liquids that indicated that their atoms liked to
coordinate themselves into regular arrangements long before the liquid was cooled to the
freezing point. The idea I took away from this conversation is that atoms
always want to be connected to each other, and if they can't do it on a grand scale, they'll settle for something more local.
His X-ray work was unpublished, and I could see why.
Referees would have a problem with this experiment, simply because it would be hard to tell whether all the liquid was at the
temperature you measured.
Control systems in those
vacuum tube days were crude, and the temperature measurement was done by inaccurate
thermocouples; so, there may have been regions of the molten mass that were actually below the melting point and should be solid. However, the old
professor was sure of his result.[1]
Scientists from
Ames Laboratory, the
University of Wisconsin-Madison, and
Iowa State University have been performing experiments that show that metallic glasses can indeed exhibit order over large scales.[2-5] One caveat is that their experiments were on just one metallic glass alloy. Another is that a
computational technique is needed to divine this order from the experimental data.[5]

To create their metallic glass, the Ames scientists used
melt spinning, a technique of alloy formation in which the molten metal is ejected in a stream onto a rapidly spinning copper wheel. Says Ames Laboratory materials scientist, Matt Kramer, "This has been one of those burning questions in material science for a while, how to describe these disordered systems. Our studies are showing this underlying structure. it's diffuse, but it's there. It's been suspected for a long time and even the general structures have been postulated, but to what degree and how to quantify them, that has been the trick."[2]
(Matt Kramer operating a scanning transmission electron microscope. US Department of Energy image).
Data for these studies were obtained with the X-ray source of the Argonne National Laboratory
Advanced Photon Source, and a technique called fluctuation
electron microscopy.[2,5] These analyses revealed that the local arrangements found in a crystalline metal are also present in the glass. The difference is that these arrangements are oriented in the crystal, but they are
randomly oriented in the glass.[2]
The current theory of metallic glasses is that they solidify from clusters of atoms with
pentagonal and
icosahedral symmetry. Pentagonal symmetry, of course, does not lead to a lattice structure.[5] Evidence for this is in the
mechanical properties of the metallic glasses. Since you can't have
dislocation movement in such structures, they don't
deform, they only break.
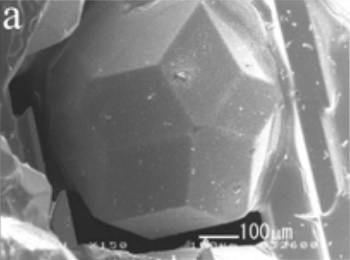
(Fig. 3a of ref. 6, via the arXiv Preprint Server).[6)]
The team's analysis of order in the the metallic glass
Zr50Cu45Al5 was unique. The fluctuation electron microscopy technique looks at subtle variations in the
electron diffraction pattern (a measure of crystallinity) as an
electron beam is scanned across a foil specimen.
Amorphous material will exhibit no place-to-place variation, so any variation would arise from crystallinity. This technique is more sensitive than conventional electron microscopy, or x-ray diffraction, in probing order in metallic glasses.[5]
These diffraction data were processed by a computer technique called "hybrid reverse
Monte Carlo simulation,"[4] and they revealed some icosahedral symmetry; and
cubic crystalline regions in nearly fifteen percent of the specimen.[5] These cluster regions tend to occur at a regular distance from each other, but they are not part of a lattice structure.[1]
So, atoms are gregarious little creatures, as I thought decades ago. The research team, encouraged by their results on Zr
50Cu
45Al
5, hope to analyze other metallic glasses.[5] The Ames Laboratory research was supported by the
U.S. Department of Energy Office of Science.[1]
References:
- This reminds me of the principle that everyone believes the results of an experiment, except the experimentalist, and the only person who believes a theoretical paper is the guy who wrote it. The person who conducts the experiment always has a way to improve it, if only he had the time and funding.
- Ames Laboratory finds ordered atoms in glass materials, Ames Laboratory Press Release, October 2, 2012.
- X. W. Fang, C. Z. Wang, S. G. Hao, M. J. Kramer, Y. X. Yao, M. I. Mendelev, Z. J. Ding, R. E. Napolitano and K. M. Ho, "Spatially Resolved Distribution Function and the Medium-Range Order in Metallic Liquid and Glass," Scientific Reports, vol. 1 (December 23, 2011), article no. 194.
- Jinwoo Hwang, Z. H. Melgarejo, Y. E. Kalay, I. Kalay, M. J. Kramer, D. S. Stone and P. M. Voyles, "Nanoscale Structure and Structural Relaxation in Zr50Cu45Al5 Bulk Metallic Glass," Phys. Rev. Lett., vol. 108, no. 19 (May 11, 2012), Document No. 195505 (5 pages).
- Kim Krieger, "Metallic Glass isn't All Glassy," APS Physics Web Site, vol. 5, no. 54 (May 11, 2012).
- Tsutomu Ishimasa, Yasushi Kaneko and Hiroshi Kaneko, "New group of stable icosahedral quasicrystals: structural properties and formation conditions," arXiv Preprint Server, August 25, 2003.
Permanent Link to this article
Linked Keywords: Scientist; baby boomer; interview; employee; scientific literature; publication record; Latin; esse quam videre; motto; Berklee College of Music; research and development; industrial research; director; laboratory; National Academy of Engineering; science; scientific; amorphous metal; metallic glass; metal alloy; crystal structure; atom; order and disorder; metal; short-range order; experiment; synchrotron X-ray source; graduate school; physical chemist; X-ray crystallography; X-ray; liquid; coordination number; freezing point; peer review; referee; temperature; control system; vacuum tube; thermocouple; professor; Ames Laboratory; University of Wisconsin-Madison; Iowa State University; computation; melt spinning; copper; materials science; Matt Kramer; scanning transmission electron microscope; US Department of Energy; Advanced Photon Source; electron microscope; electron microscopy; randomness; pentagon; pentagonal; icosahedral symmetry; icosahedron; symmetry; mechanics; mechanical properties; dislocation; plasticity; deformation; triacontahedron; Zinc; Magnesium; Mg; Scandium; Sc; dodecahedron; cube; tetrahedron; arXiv; Zirconium; Zr; Copper; Cu; Aluminium; Al; electron diffraction; cathode ray; electron beam; amorphous solid; amorphous material; Monte Carlo method; cubic crystal system; U.S. Department of Energy Office of Science.