Flow Batteries
July 18, 2012
An
electrochemical battery has its
energy stored in the
potential energy of the
chemical reactions it contains. In a
lead-acid battery, the net
discharge reaction is
Pb + PbO2 + 2H2SO4 --> 2PbSO4 + 2H2O
The reaction has an
enthalpy (ΔH°) of -549
kJ, per starting
mole of
lead (Pb); the negative sign indicating a reaction in which we get some energy. Lead and
lead dioxide (PbO2) are somewhat heavy, so it takes quite a bit of
mass to get this energy. There is, however, a lot of energy to be had. Since a
watt is a joule per second, 549 kJ is about 150
watt-hour. In theory, a ten
pound battery could run a
hair dryer for about half an hour.
The
volumetric energy density is constrained by the
volume of the
sulfuric acid (H2SO4), which is in a
water solution. Right away, we take a volume penalty, since the 4.2
Molar sulfuric acid used is just a third H
2SO
4, and two-thirds water. Although lead-acid cells are rechargeable, once they're charged, that's the limit of your energy storage. Any extra energy you have can't be stored, so it's lost.
Instead of having huge electrochemical batteries, wouldn't it be nice if we could have a smaller device that converts the energy into a liquid without consuming the
electrodes in the process? We could then pump, or pour, this energy into suitable storage vessels, and then put this liquid energy back into the cell when we need to extract some power. This is reminiscent of the
energon cubes in the
Transformers animated television series.
This
science fiction is actually fact in a device called a
flow battery; specifically, a
redox flow battery. All the chemical reactions are contained in the solution, and the electrode materials are not consumed. The power you can get from such a flow battery depends in the electrode area, so our battery still needs some heft. However, the fluid volume depends only on the capacity of our storage tanks; and, the cost of a tank becomes less per unit volume as the tank volume increases.
Flow batteries are a possible solution to
load balancing in
renewable energy systems, such as
wind and
solar energy. Such energy sources function intermittently, at the whim of
nature, so you need to store energy
while the sun shines, so to speak, and use it at a later time. Flow batteries eliminate the need to have a multitude of cells, formed from expensive
materials, in a
parallel combination.
A team of
scientists from
Drexel University's A.J. Drexel Nanotechnology Institute has applied the flow battery concept to
supercapacitors. In this case, the
electrolyte is replaced by a
slurry of
carbon particles and electrolyte. Just as in standard supercapacitors, the energy is stored in an
electric double layer at the charged carbon particles.[1-2]
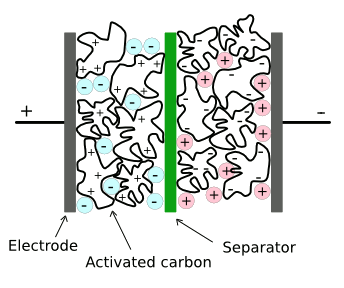
Structure of a conventional supercapacitor.
There are separate ionic liquids that are negatively and positively charged. One disadvantage of current materials is that these ionic liquids decompose at voltages above a few volts.
(Via Wikimedia Commons, modified))
This research project was led by
Yury Gogotsi, director of the
A.J. Drexel Nanotechnology Institute. I wrote about some of Gogotsi's previous work with supercapacitors in a
previous article (Carbon Nano-Onion Supercaps, August 26, 2010). This flow capacitor research is published as an article in the July, 2012, issue of
Advanced Energy Materials, a special issue devoted to battery materials.[1]
As can be seen from the diagram, below, the device is simply made, with separate reservoirs for
electron donor (+) and
electron acceptor (-) materials. The flow materials are thick slurries of small carbon particles in the electrolyte liquids.[1-3] Uncharged, or partially-charged, slurry is pumped through the flow cell, which charges the carbon particles and deposits the charged slurry into reservoirs. When energy is needed, the process is reversed, and the charge flows through the external circuit attached to the flow capacitor.[2]
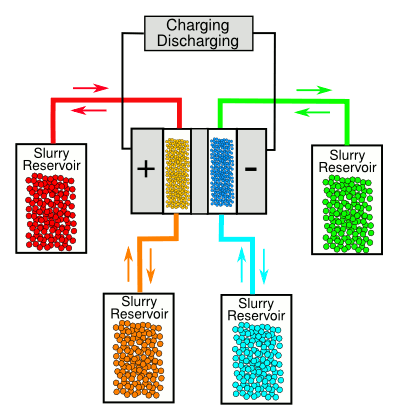
The Drexel University flow battery.
(Diagram rendered by the author using Inkscape)
If conventional supercapacitors can be a guide, such a system would be robust and long-lived, since they do not have any performance degradation for a million
charge-discharge cycles.[2] There is still considerable work needed to make a battery system that's competitive with other
energy storage options. Gogotsi's team is working on development of slurry compositions composed of a variety of carbon
nanomaterials and electrolytes.[2] Said Gogotsi,
“We have observed very promising performance so far, being close to that of conventional packaged supercapacitor cells... However, we will need to increase the energy density per unit of slurry volume by an order of magnitude, and achieve it using very inexpensive carbon and salt solutions to make the technology practical."[2]
References:
- Volker Presser, Christopher R. Dennison, Jonathan Campos, Kevin W. Knehr, Emin C. Kumbur and Yury Gogotsi, "Electrochemical Flow Cells: The Electrochemical Flow Capacitor: A New Concept for Rapid Energy Storage and Recovery," Advanced Energy Materials, vol. 2, no. 7 (July, 2012), pp. 895-902.
- Britt Faulstick, "Making "Renewable" Viable: Drexel Engineers Develop New Technology for Grid-Level Electrical Energy Storage," Drexel University Press Release, July 10, 2012.
- Supplemental videos for ref. 1, Advanced Energy Materials, vol. 2, no. 7 (July, 2012).
Permanent Link to this article
Linked Keywords: Electrochemical battery; energy; potential energy; chemical reaction; lead-acid battery; discharge; enthalpy; joule; kJ; mole; lead (Pb); lead dioxide (PbO2); mass; watt; watt-hour; pound; hair dryer; volumetric energy density; volume; sulfuric acid (H2SO4); aqueous solution; water solution; Molar concentration; Molar; electrode; energon cube; Transformers; science fiction; flow battery; redox; electrical load balancing; renewable energy; wind power; solar energy; nature; Make hay while the sun shines; material; parallel circuit; scientist; Drexel University; A.J. Drexel Nanotechnology Institute; supercapacitor; electrolyte; slurry; carbon; electric double layer; Wikimedia Commons; Yury Gogotsi; Advanced Energy Materials; electron donor; electron acceptor; Inkscape; charge-discharge cycle; energy storage; nanomaterial.