Carbon at Earth's Core
December 7, 2012
I once joked that a
physicist is the type of
scientist who tries to find
steel in the
Periodic Table. Since most physicists deal with the immaterial, such as
fields, they can be excused. It's only their
solid state brethren who work with actual
materials. Steel, of course, is an
alloy of
carbon and
iron, and this useful material has been with us for several centuries.
The
Iron Age, the last of the
three ages, began at about 1000
BC. The Iron Age was preceded by the
Stone Age and the
Bronze Age. There is no precise dating for the Iron Age, since iron foundry started at different times in different regions of the world. This first iron contained little carbon, so none of it was steel in the modern sense. Steel is essentially a product of the
Industrial Revolution.
Metallurgy is so important to
civilization that the
ancients identified the
Ages of Man with the succession of
metals in common use; principally,
gold->
silver->
bronze->iron.
Hesiod, in his
Works and Days, saw a parallel between the progression from noble to base metal and the apparent debasing of human existence (see figure).

"For now truly is a race of iron, and men never rest from labor and sorrow by day, and from perishing by night; and the gods shall lay sore trouble upon them." [Hesiod, Works and Days, ll. 176-178, refs. 1-2]. (Via Project Perseus))
Carbon
atoms (
atomic radius = 70 pm) are much smaller than iron atoms (atomic radius = 140 pm). Since their atomic radii are in a 1:2 ratio, the atomic volumes differ by a factor of eight. This size difference allows carbon atoms to populate the small
interstices of the iron
body-centered cubic crystal lattice (
ferrite), but not to too great an extent. The maximum
solid solubility of carbon in ferrite is just 0.02
weight-%, as the
phase diagram shows. Carbon is still very
miscible in liquid iron.
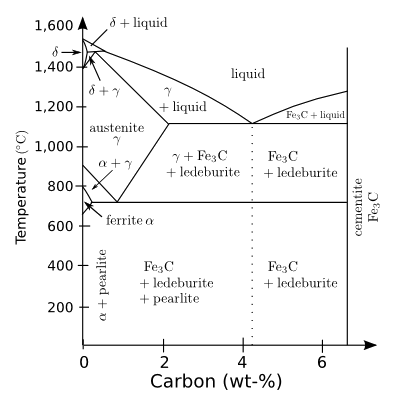
The iron-rich portion of the iron-carbon phase diagram.
The maximum solid solubility of carbon in iron is just 0.02 weight-%.
(Figure by Christophe Dang Ngoc Chan, slightly modified, via Wikimedia Commons)
Such phase diagrams represent alloys at
standard pressure (about 1
atmosphere); and, since most solid components of alloys are quite
incompressible, they're a good representation for even higher pressures. The
center of the Earth is another environment altogether, with a temperature of about 5430
°C and a pressure of about 350
gigapascals (3,450,000 atmospheres).
Because of the high pressure, the inner core of the Earth is solid. It's surrounded by an
outer core of liquid. The inner and outer cores are composed primarily of iron with about 4%
nickel. These two metals together don't quite account for the core's
density, so it's presumed that there are some lighter
elements dissolved. There are
chemical reasons to suppose that
oxygen and
sulfur are the lighter elements. It's very difficult to remove oxygen and sulfur from iron.
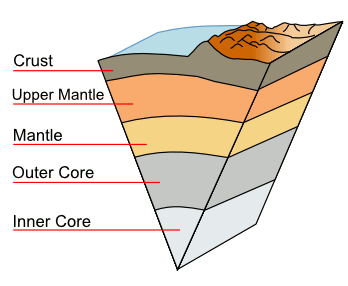
A cutaway diagram of the Earth, showing the core and other components.
This diagram, except for the surface features, is to scale.
(Modified Wikimedia Commons image)
Various studies have been done in an attempt to estimate the carbon concentration of Earth's core. Concentration estimates for carbon in iron at relevant pressures and temperatures have ranged from about 7.5 wt.-% (2410 °C, 2 GPa) for the liquid, outer core,[3] to 2.6-3.7 wt.-% for the solid, inner core.[4] This open question has prompted a recent
first-principles molecular dynamics study that gives a far lower estimate of 0.1–0.7 wt-% for carbon concentration in the inner core.[5-6]
This study, published in a recent issue of the
Proceedings of the National Academy of Sciences by
Yigang Zhang of the
Chinese Academy of Sciences (
Beijing, China) and
Qing-Zhu Yin of the
Department of Geology,
University of California (Davis, California), looks particularly how easily carbon will dissolve in
silicate, as found in
Earth's crust, as opposed to the iron of Earth's core.[5]
The quantity of core carbon is important to the composition of Earth's crust, since the presence of carbon would have determined how much of other light elements would have dissolved in the core. What was dissolved in the core would not have been present for crustal formation.[5]
References:
- Greek text from Project Perseus; source, Hesiod, "Works and Days," from The Homeric Hymns and Homerica with an English Translation by Hugh G. Evelyn-White (Harvard University Press, Cambridge, MA., and William Heinemann Ltd., London), 1914.
- English translation of ref. 1.
- Rajdeep Dasgupta and David Walker, "Carbon solubility in core melts in a shallow magma ocean environment and distribution of carbon between the Earth's core and the mantle," Geochimica et Cosmochimica Acta, vol. 72 (2008), pp. 4627-4641 (PDF file).
- Zulfiya G. Bazhanova, Artem R. Oganov and Omar Gianola, "Fe-C and Fe-H systems at pressures of the Earth's inner core," arXiv Preprint Server, June 3, 2012.
- Yigang Zhang and Qing-Zhu Yin, "Carbon and other light element contents in the Earth's core based on first-principles molecular dynamics," Proc. Natl. Acad. Sci., vol. 109 no. 48 (November 27, 2012), pp. 19579-19583
- Earth's Core & Carbon: Planetary Center Holds Largest Store Of Common Element, Computer Simulation Suggests, Our Amazing Planet, via Huffington Post, November 23, 2012.
Permanent Link to this article
Linked Keywords: Physicist; scientist; steel; Periodic Table; field; solid-state physics; material; alloy; carbon; iron; Iron Age; three ages; Anno Domini; BC; Stone Age; Bronze Age; Industrial Revolution; metallurgy; civilization; Ancient Greece; ancients; Ages of Man; metal; gold; silver; bronze; Hesiod; Works and Days; Project Perseus; atom; atomic radius; interstitial compound; interstice; body-centered cubic; crystal lattice; ferrite; solid solubility; weight-%; phase diagram; miscibility; miscible; Wikimedia Commons; standard pressure; atmosphere; compressibility; incompressible; inner core; center of the Earth; Celsius; °C; gigapascal; outer core; nickel; density; chemical element; chemistry; oxygen; sulfur; first-principles; molecular dynamics; Proceedings of the National Academy of Sciences of the United States of America; Yigang Zhang; Chinese Academy of Sciences; Beijing, China; Qing-Zhu Yin; Department of Geology; University of California (Davis, California); silicate mineral; silicate; Earth's crust; Hugh G. Evelyn-White.