Supercooled Water
December 22, 2011
I've used
chemical thermodynamics very often to predict what will happen when
materials are combined at a given
temperature. When I presented my results, I was always careful to point out that
thermodynamics gives answers for
systems in equilibrium, and the
kinetics of a
reaction are another thing altogether.
You only have to mix
hydrogen and
oxygen together to see that this is the case. Nothing happens, unless an
electrical spark or a
catalytic surface is introduced into the system. When that happens, you're quite convinced that the predicted reaction takes place.
Water has many unusual properties, so many that there's a web site dedicated to them.[1] These unusual properties are likely the result of water being small
molecule, and the forces holding these molecules together in a
liquid or
solid coming from
hydrogen bonding. There are sixteen solid phases of
water ice, named
ice Ih (our "normal" ice) to
ice XV, existing at different temperatures and
pressures, as shown in the figure.
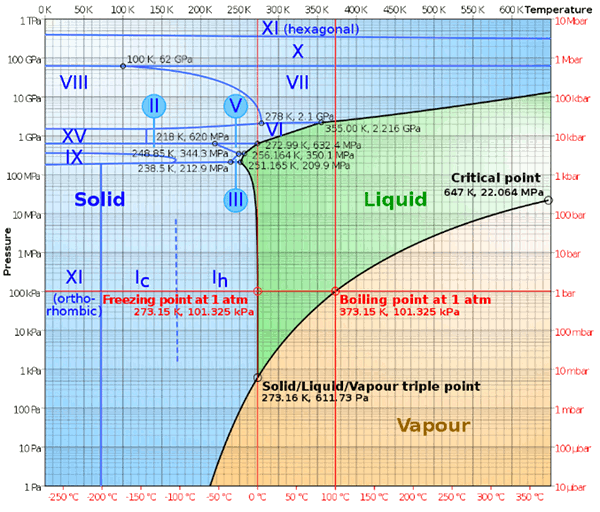
Temperature-pressure phase diagram of water. Ice IV and Ice XII are metastable, so they are missing from this equilibrium diagram. (Image by Cmglee, via Wikimedia Commons).
It's well-known that liquids can be
supercooled; that is, they can be cooled below their
freezing points without
solidifying. Water can be supercooled, so a fair
scientific question is how far can water be supercooled, and what are the factors that govern the degree of supercooling. These questions were addressed in a recent study by
Valeria Molinero, an
assistant professor of
chemistry at the
University of Utah (Salt Lake City, Utah), and her
doctoral student, Emily B. Moore.[2-3]
The properties of supercooled water have been investigated
experimentally, but only at the lowest temperature of -42°F (232 K). This is the
homogeneous nucleation temperature. Below this temperature,
crystallization of water is too rapid for the properties of the remaining liquid to be measured.[3] For this reason, the Utah chemists used computers at the University of Utah's
Center for High Performance Computing instead of
test tubes.[3] Supercooled water was both
simulated at the molecular level and
modeled using experimental data.[3]
An important first step in their research was optimization of the
computer codes used in such modeling and simulation. They were able to speed computations by a factor of 200, but computation still took thousands of hours of computer time on their simulation of freezing of a 32,768 molecule cluster. The number 32,768, of course, is 2
15.
They calculated the
heat capacity,
density and
compressibility of water as it is supercooled; and they also simulated the rate of ice crystallization.[3] Their calculations extended to -55°F, the temperature at which the maximum crystallization rate is obtained, and also the lowest possible supercooled temperature.[3]
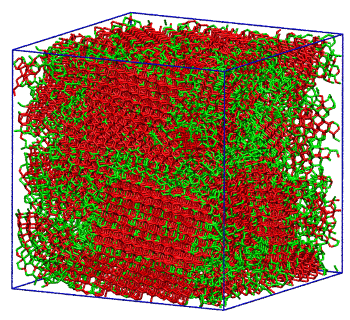
Molecular simulation of water crystallization from supercooled water. Regular ice is shown in red, and "intermediate ice" is shown in red.
(University of Utah image))
So, exactly how does supercooled water freeze? As water cools, its structure becomes more and more like that of ice. As water approaches -55°F, there's a large increase in the proportion of water molecules that bond with others into a
tetrahedron, an assemblage of molecules very much like that in ice. Molinero calls this phase "intermediate ice."[3] The calculations showed that
amorphous ice, which is
glassy ice formed in a cooling process too rapid to allow crystallization, is about 25% intermediate ice and small ice crystals.[3]
All of this molecular dynamics is reflected in the thermodynamics. At -55°F, the density decreases, and the heat capacity increases, as does the compressibility.[3] The heat capacity and compressibility of supercooled water would become
infinite at ~225 K (-55.4°F), so there's a connection between these thermodynamic quantities and the crystallization rate of ice.[2]
References:
- Martin Chaplin, "Anomalous properties of water," London South Bank University Web Site.
- Emily B. Moore and Valeria Molinero, "Structural transformation in supercooled water controls the crystallization rate of ice," Nature, vol. 479, no. 7374 (November 24, 2011), pp. 506-508.
- Lee J. Siegel, "Supercool - Utah Chemists: Water Doesn't Have to Freeze until -55 F," University of Utah Press Release, November 23, 2011.
Permanent Link to this article
Linked Keywords: Chemical thermodynamics; material; temperature; thermodynamics; thermodynamic equilibrium; kinetics; reaction; hydrogen; oxygen; electrical spark; catalytic surface; water; molecule; liquid; solid; hydrogen bonding; water ice; pressure; phase diagram; metastable; Wikimedia Commons; supercooling; supercooled; melting point; freezing point; freezing; solidification; scientific; Valeria Molinero; assistant professor; chemistry; University of Utah (Salt Lake City, Utah); Doctor of Philosophy; experiment; homogeneous nucleation; crystallization; Center for High Performance Computing; test tube; computer simulation; computer; model; computer code; heat capacity; density; compressibility; tetrahedron; amorphous ice; glass; infinite.