Pyrite Photovoltaics
January 24, 2011
Iron pyrite, FeS
2, is a common mineral on Earth because of the ubiquity of both iron and sulfur. It's called "fool's
gold" because its color resembles gold's luster. The name, "pyrite," derives from the
Greek, πυρ (pyr), the word for
fire. It was named pyrite, since sparks are generated when pyrite mineral specimens are struck by
steel.
Pliny the Elder described this sparking effect in his
Natural History.[1] This is a reaction with
oxygen in air to form more stable
ferrite,
4 FeS2 + 11 O2 -> 2 Fe2O3 + 8 SO2
This reaction is often used in introductory
chemistry courses to illustrate that most reactions are not as simple as "two parts
hydrogen plus one part oxygen."
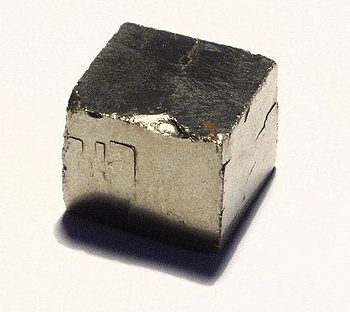
All that glitters is not gold. A pyrite cube. (Photo by Richard Wheeler).
Pyrite is not that interesting chemically, but it has one property that endears it to
physicists. It's a
semiconductor, and it was used, as was its close cousin,
galena, a crystal form of
lead sulfide, as a
detector crystal in
early radio receivers. In these
cat's whisker detectors, a wire made contact with an appropriately sensitive area of the mineral
crystal to form a
Schottky diode. These detectors were the first
semiconductor devices, although their operating principle wasn't known until the development of
solid state physics in the mid-
twentieth century. The performance was close to that of the formerly ubiquitous, but now scarce, 1N34A
germanium diode.
Not only is pyrite interesting as a rectifier, it will function as a
photovoltaic diode with a
bandgap of 0.95 eV. This is a good value, quite close to that of
silicon (see table). Bandgap is an important property for a
photovoltaic device, since only
photons with energy greater than the bandgap can produce a
current. The portion of the
solar spectrum that will produce a current in a material is lower in
wavelength than the values shown in the table. In the case of pyrite, nearly all the solar radiation is utilized.[3]
Germanium is not that useful as a photovoltaic, since its properties are highly dependent on temperature. That's why
silicon is used in today's transistors, and not the original germanium.
If you could overcome the obvious deterioration from environmental exposure, could you use pyrite as a solar cell material? It would be difficult to
prepare large single crystals of pyrite, and such an expensive process would negate any savings we might have on the raw material. One other problem with iron pyrite crystals is that they're usually loaded with defects which limit conductivity.[4]
A research group at the
University of California (Irvine) has been investigating production of
nanocrystalline iron pyrite for thin-film solar cells.[4-5] In a recent paper in the
Journal of the American Chemical Society [4], a venue chosen because the paper is more about the
material than a solar cell, they describe a process for making high quality
nano-colloidal inks of iron pyrite. Using the inks, they prepared
polycrystalline pyrite thin films by
sintering at 500-600
oC in a
sulfur atmosphere. The
National Science Foundation has provided three years of funding for the UC-Irvine group.[4]
Nor is UC-Irvine the only group working with pyrite. A group from the
University of California (Berkeley) and
Lawrence Berkeley National Laboratory has investigated
hydrothermal synthesis of pyrite nanocrystals.[6-7] This group was apparently the first to synthesize pyrite nanocrystals. The nanocrystal size range is 100 to 500 nm.[8] Says Berkeley team member Cyrus Wadia,
"The theoretical efficiency of iron sulfide is 31 percent. That's as good as silicon.. What's more, 20 nanometers of pyrite can absorb as much light as 300 micrometers of silicon."[8]
The Berkeley group is also investigating the use of
copper sulfide as a complementary
p-type material for use with
n-type pyrite. Paul Alivisatos, one of the co-investigators on these studies, has founded a company, (
Solexant,
San Jose, CA), to develop low-cost solar cells based on nanomaterials. Solexant will use a printing technique with a goal of 10 percent efficient, $1/watt devices.[9] Solexant hasn't disclosed its materials, but they do say that their inks are suspensions of rod-shaped nanocrystals, four nanometers in diameter by 20-30 nm in length. Solexant is using a roll-to-roll process, which ensures an inexpensive final product. Pieces from the flexible rolls are then bonded to glass. Their future direction is towards multilayer, broad-spectrum cells.
References:
- John Bostock (Trans.), "Pliny the Elder, The Natural History," Taylor and Francis (London, 1855); Book XXXVI. The Natural History Of Stones. Chap. 30.— Molar Stones. Pyrites; Seven Remedies. (Latin Text)
"Some writers mention another kind of pyrite also. Those among them have the greatest affinity to fire which we distinguish as "live" pyrites. They are the most ponderous of all, and are found remarkably useful for advance-guards when laying out encampments; for, on being struck with a nail or any other kind of stone, they emit a spark, which, received upon sulphur, dried fungus, or leaves, produces a fire almost sooner than it could be named."
"Pyritarum etiamnum unum genus aliqui faciunt plurimum ignis habentis. quos vivos appellamus, ponderosissimi sunt, hi exploratoribus castrorum maxime necessarii. qui clavo vel altero lapide percussi scintillam edunt, quae excepta sulpure aut fungis aridis vel foliis dicto celerius praebet ignem."
- Iron pyrite page on Wikipedia.
- The Renewable Resource Data Center, "Reference Solar Spectral Irradiance: Air Mass 1.5."
- Tiffany Hsu, "Fool's Gold Catches Eye Of Solar Energy Researchers," Los Angeles Times, January 14, 2011.
- James Puthussery, Sean Seefeld, Nicholas Berry, Markelle Gibbs, and Matt Law, "Colloidal Iron Pyrite (FeS2) Nanocrystal Inks for Thin-Film Photovoltaics," J. Am. Chem. Soc., DOI: 10.1021/ja1096368, December 22, 2010.
- Cyrus Wadia, Yue Wu, Sheraz Gul, Steven K. Volkman, Jinghua Guo and A. Paul Alivisatos, "Surfactant-Assisted Hydrothermal Synthesis of Single phase Pyrite FeS2 Nanocrystals," Chem. Mater., vol. 21, no. 13 (June 16, 2009), 2009, 21 (13), pp 2568-2570.
- Cyrus Wadia, A. Paul Alivisatos and Daniel M. Kammen, "Materials Availability Expands the Opportunity for Large-Scale Photovoltaics Deployment," Environ. Sci. Technol. vol. 43, no. 6 (February 13, 2009), pp 2072-2077.
- "Mining fool's gold for solar: Cyrus Wadia is using abundant materials to grow nanocrystals for cheaper photovoltaics,"Technology Review, November 1, 2009 (via ecnext.com).
- Katherine Bourzac, "Thin-Film Solar with High Efficiency," Technology Review, November 19, 2009.
Permanent Link to this article
Linked Keywords: Iron pyrite; gold; Greek language; fire; steel; Pliny the Elder; Natural History; oxygen; iron(III) oxide; ferrite; chemistry; hydrogen; Richard Wheeler; physicist; semiconductor; galena; lead sulfide; detector crystal; early radio receivers; cat's whisker detectors; crystal; Schottky diode; semiconductor device; solid state physics; twentieth century; germanium diode; photodiode; photovoltaic diode; bandgap; silicon; photovoltaic; photon; electric current; solar spectrum; wavelength; germanium; silicon; electronvolt; eV; nanometer; nm; Iron Pyrite; Gallium Arsenide; Silicon Carbide; Czochralski process; University of California (Irvine); nanocrystalline; Journal of the American Chemical Society; materials science; nano-colloid; polycrystalline; sintering; sulfur; National Science Foundation; University of California (Berkeley); Lawrence Berkeley National Laboratory; hydrothermal synthesis; copper sulfide; p-type; n-type; Solexant; San Jose, CA.