Lithium Ion Electrodes
December 13, 2011
Silicon, the miracle element of our present electronic age, is quickly being supplanted by its neighbor,
carbon, in the
periodic table of elements. One advantage that carbon has over silicon is that it exists in several
alloptopic forms.
Portion of the periodic table of elements near carbon and silicon.
(Via Wikimedia Commons))
There's
diamond;
graphite, which, as a single atomic layer is called
graphene; and the various
fullerenes, including
carbon nanotubes. There's also
glassy carbon, useful as an
electrode material in
electrochemistry, and for high temperature
crucibles. Diamond has high
thermal conductivity, so it's useful as a
substrate material. Although it's hard to make
transistors from diamond, some very high performance transistors have been made from graphene and carbon nanotubes.
One current use of carbon in its graphitic form is as an electrode material for
lithium-ion batteries, the ubiquitous rechargeable batteries that are contained in much of our consumer electronics. In fact, our mobile computing experience would be quite different without them, since their
energy density allows their very small size. That's why we have
cigarette package-sized
cellphones, and not cigarette carton-sized cellphones.
The graphite electrode in these batteries is there to accept the lithium ions that are produced in the reaction that reduces the typical
lithium cobalt oxide (LiCoO
2) cathode. The graphite electrode serves the dual purpose of being a good
electrical conductor, and allowing lithium ions to populate the region between the graphite
atomic planes of the graphic
crystal structure, a process called
intercalation. Motion of lithium ions between graphite planes is rapid, so the batteries can be discharged and recharged easily.
Still, everyone wants cellphones with longer life between charges, and this desire is becoming all the more important with the advent of
smartphones that use considerable amounts of power. Silicon absorbs lithium to a greater extent than graphite; in fact, silicon has the highest theoretical
charge capacity, 4.2 ampere-hours/gram. Graphite has a capacity of about 0.4
ampere-hours/
gram. However, silicon is not that conductive, and the silicon expands by 400% after absorption of lithium. The volume expansion leads to
mechanical failure of silicon electrodes.
As a first practical attempt at using silicon as a lithium battery electrode, researchers at
Stanford University and their colleagues prepared silicon
nanowires on conductive substrates to circumvent both the conductivity and volume expansion issues. They achieved 75% of the theoretical capacity.[1]
Another silicon electrode geometry, invented at the
Hanyang University (Ansan, South Korea), consists of silicon nanoribbons riddled with holes to allow for expansion. The South Korean electrode exhibited a 2.4 ampere-hours/gram capacity after a hundred cycles.[2-3]
Another approach by scientists at the
Georgia Institute of Technology combines graphite and silicon into a composite electrode material.[4-5] Their process produces a structure that resembles silicon nano-apples, 10-30 μm in diameter, hanging from graphite branches. The carbon tree-like structure is formed from
carbon black nanoparticles that are
annealed at high temperature. The silicon nanospheres are formed using
chemical vapor deposition.[5]
This is an open structure that allows the
electrolyte to make contact with the silicon for quick charging and discharging. The open structure also allows room for expansion of the silicon.[5] Particles of this branched structure are pressed together to form the battery electrode. Coin-sized composite electrodes demonstrated a capacity of 1.95 ampere-hour/gram, which is more than five times the theoretical capacity of graphite.[4-5]
A somewhat different approach to a carbon-silicon composite electrode is being investigated by a team of scientists led by
Harold H. Kung, a professor of
chemical and biological engineering at
Northwestern University's Robert R. McCormick School of Engineering and Applied Science. Their approach is to sandwich silicon nanospheres between graphene flakes that are prepared with defects that allow easy passage of the lithium ions (see figure).[6-8]
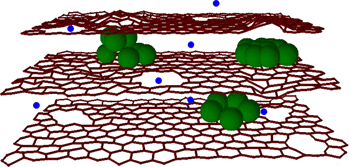
A graphene-silicon composite anode for lithium ion batteries.
(Via Northwest University))
Says Kung,
"We have found a way to extend a new lithium-ion battery's charge life by 10 times... Even after 150 charges, which would be one year or more of operation, the battery is still five times more effective than lithium-ion batteries on the market today."[7-8]
One advantage that the graphene flakes have over graphite is that the lithium ions have a shorter travel time from the edge of the carbon to any region within. The defects in the graphene flakes, the 10-20 nm holes that allow a transverse flow of lithium through the flakes, are made by
chemical oxidation.[7-8]
Now that these lithium-ion electrodes, used as
anodes in rechargeable batteries, have been optimized nearly as much as possible, it's time to concentrate on the
cathode. Says Stanford's Yi Cui, who developed the silicon nanowire anode,[1]
"We are actually limited more by the cathode... Improving the anode will have a very big impact. But improving the cathode can have an even larger impact."[3]
References:
- Candace K. Chan, Hailin Peng, Gao Liu, Kevin McIlwrath, Xiao Feng Zhang, Robert A. Huggins and Yi Cui, "High-performance lithium battery anodes using silicon nanowires," Nature Nanotechnology, vol. 3, No. 1 (January, 2008), pp. 31-35.
- Hyunjung Kim, Byunghee Han, Jaebum Choo, Prof. and Jaephil Cho, Prof., "Three-Dimensional Porous Silicon Particles for Use in High-Performance Lithium Secondary Batteries," Angewandte Chemie, vol. 120, no. 52 (December 15, 2008), pp. 10305-10308.
- Peter Fairley, "Realizing Lithium-Battery Potential - Nanoporous silicon that soaks up ions without self-destructing can make better batteries," Technology Review, December 3, 2008.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, "High-performance lithium-ion anodes using a hierarchical bottom-up approach," Nature Materials, vol. 9, no. 4 (April, 2010), pp. 353-358.
- Darren Quick, "Silicon-based anode to boost lithium-ion battery performance," Gizmag, April 8, 2010.
- Xin Zhao, Cary M. Hayner, Mayfair C. Kung and Harold H. Kung, "In-Plane Vacancy-Enabled High-Power Si–Graphene Composite Electrode for Lithium-Ion Batteries," Advanced Energy Materials, vol. 1, no. 6 (November, 2011), pp. 1079-1084.
- Megan Fellman and Sarah Ostman, "Better Batteries - New technology improves both energy capacity and charge rate in rechargeable batteries," Northwest University Press Release, November 14, 2011.
- Sarah Ostman, "Researchers Design Rechargeable Battery with Improved Charge Capacity, Rate," Northwest University McCormick School of Engineering and Applied Science Press Release, November 2, 2011.
Permanent Link to this article
Linked Keywords: Silicon; periodic table of elements; carbon; alloptopic form; Wikimedia Commons; diamond; graphite; graphene; fullerene; carbon nanotube; glassy carbon; electrode; electrochemistry; crucible; thermal conductivity; substrate; transistor; lithium-ion battery; energy density; cigarette; cellphone; lithium cobalt oxide; electrical conductor; atomic; crystal structure; intercalation; smartphone; charge capacity; ampere-hours; gram; mechanical failure; Stanford University; nanowire; Hanyang University (Ansan, South Korea); Georgia Institute of Technology; carbon black; annealing; chemical vapor deposition; electrolyte; Harold H. Kung; chemical and biological engineering; Northwestern University; Robert R. McCormick School of Engineering and Applied Science; chemical oxidation; anode; cathode.