William Lipscomb
April 18, 2011
When I was a
graduate student, there was a cartoon posted on one of the
chemistry bulletin boards. It was entitled, "A Boron Chemist's Version of the Periodic Table." It was a
Periodic Table, but it was drawn so that the
boron square filled as much area as the rest of the table. As a putative
metallurgist, I didn't see what was that interesting about boron, except for the fact that the chemistry building was once evacuated because of a leaky
boron trifluoride BF
3 gas cylinder. All the action seemed to be at
carbon, boron's next door neighbor.
William Nunn Lipscomb, an important figure in
boron chemistry, died on April 14, 2011, at age 91.[1-4]

William Nunn Lipscomb, circa 1980.
Photo by James S. Lipscomb, via Wikimedia Commons
William Nunn Lipscomb, Jr., was born in
Cleveland, Ohio, on December 9, 1919, but he was raised in
Lexington, Kentucky. Because of his
Kentucky upbringing, Lipscomb was affectionately called "
The Colonel." As is common behavior for budding
scientists, this author included, Lipscomb assembled a home
laboratory as a teen, making the usual assortment of
foul-smelling chemicals and
fireworks. Lipscomb had amassed a considerable quantity of
labware, and he donated his equipment to his
high school at graduation.[2] His family had a modest income, so Lipscomb needed to attend the inexpensive local university, the
University of Kentucky, surprisingly on a
clarinet scholarship.[2] Lipscomb received a
Bachelor of Science degree in Chemistry from the University of Kentucky in 1941.
What is most interesting to me is that Lipscomb almost became a
physicist. He spent one year studying physics at
Caltech before switching to physical chemistry. Lipscomb was awarded a
Ph. D. in Chemistry from Caltech in 1946. He then taught at the
University of Minnesota for an extended period through 1959, when he became a professor of chemistry at
Harvard University. At Harvard, he was Abbott and James Lawrence Professor of Chemistry from 1971 to 1990, and Abbott and James Lawrence Professor of Chemistry Emeritus from 1990.[1]
Linus Pauling was Lipscomb's thesis advisor at Caltech, and Pauling's ideas influenced him as they did most chemists. Lipscomb, however, thought that Pauling's ideas about boron bonding were wrong. One problem in determining the chemistry of boron was that very few boron compounds existed; and the ones that did, such as boron trifluoride (BF
3) that I mentioned earlier, had the
expected bonding.
There are several stable
inorganic compounds that contain boron.
Boranes, which are compounds of boron and
hydrogen, are not as stable, and some are
explosive. Many boron compounds are so
reactive that
experiments must be done in
vacuum. As Lipscomb recalled in his
Nobel Prize speech,[5]
The subsequent low temperature studies of single crystals of these volatile and unstable boranes were not without hazards. Vacuum line techniques were learned as we needed them. Fortunately, no serious injuries were incurred as a result of several explosions resulting from cracks in these vacuum systems. I was relieved, on one occasion, when I had taken Russell Grimes to a hospital in Cambridge after one of these explosions to hear the doctor tell me, "Louis Fieser sends me much more interesting cases than you do."
Fieser was a Harvard organic chemist who was involved with a
wartime project to have
bats with timed incendiary charges start fires in
Japan.
During
World War II, Lipscomb did military research during the day and his graduate research at night. Lipscomb's doctoral dissertation was locked in a safe for several years because it contained classified research.[2]
In order to elucidate the bonding structures of the boranes, Lipscomb and his students would
crystallize them at low temperature and perform
X-ray crystallographic analysis. One early, and quite unusual discovery, was that the boron atoms in some boranes were bonded together by a hydrogen atom. Until that time, bonds were considered to be possible only when atoms shared an
electron pair. His seminal paper, "The Valence Structure of the Boron Hydrides," coauthored with Bryce Crawford Jr. and W. H. Eberhardt, appeared in 1954.[6] Even Pauling was impressed.[7]
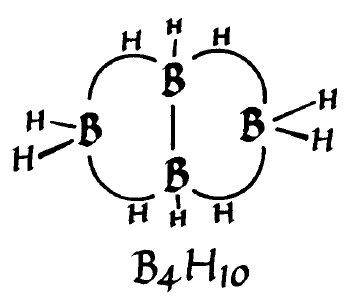
Drawing of the structure of B4H10 from William Lipscomb's Nobel Prize lecture.
(Nobel Prize Foundation)
Lipscomb was awarded the 1976
Nobel Prize in Chemistry for "his studies on the structure of boranes illuminating problems of chemical bonding." Two of Lipscomb's students were also awarded chemistry Nobels; namely,
Roald Hoffmann (1981) and
Thomas Steitz (2009).
One interesting item, as revealed in an interview with Lipscomb in 2001, is that he lost
NSF funding shortly after receiving the Nobel Prize because the NSF thought that boranes were a dead field.[8] This forced him to change his research field to
biochemistry, where he worked on the structure determination of
proteins using the crystallographic techniques that had served him well for the boranes.
Lipscomb played clarinet throughout his life. Like
Richard Feynman, who was also from Caltech, he was a
practical joker.
References:
- William Lipscomb dies at 91 - Harvard professor won Nobel Prize in chemistry, Campus & Community Obituaries, Harvard University Web Site, April 15, 2011.
- By Thomas H. Maugh II, "William N. Lipscomb dies at 91; won Nobel Prize in chemistry," Los Angeles Times, April 16, 2011.
- Mark Feeney, "Nobel laureate and Harvard professor dead at 91 - William N. Lipscomb researched boranes," The Boston Globe, April 16, 2011
- William Lipscomb page on the Nobel Prize Web Site.
- Nobel Prize Lecture by William Lipscomb, Nobel Prize Web Site, 1976.
- W. H. Eberhardt, B. Crawford, Jr. and W. N. Lipscomb, "The Valence Structure of the Boron Hydrides," J. Chem. Phys. vol. 22, no. 6 (June 1, 1954), pp. 989-1001.
- Linus Pauling, Letter to Crawford, Eberhardt, Lipscomb, OSU Special Collections, March 30, 1954.
- Interview with Professor William N. Lipscomb by Joanna Rose, Nobel Prize Web Site, December 3, 2001.
Permanent Link to this article
Linked Keywords: Graduate student; chemistry; Periodic Table; boron; metallurgist; boron trifluoride; carbon; William Nunn Lipscomb; boron chemistry; James S. Lipscomb; Wikimedia Commons; Cleveland, Ohio; Lexington, Kentucky; Kentucky; Kentucky Colonel; scientist; laboratory; hydrogen sulfide; foul-smelling chemicals; fireworks; laboratory glassware; labware; high school; University of Kentucky; clarinet; scholarship; Bachelor of Science; physicist; California Institute of Technology; Caltech; Doctor of Philosophy; Ph. D.; University of Minnesota; Harvard University; Linus Pauling; Lewis acids and bases; inorganic compounds; boranes; hydrogen; explosive; reactive; experiment; vacuum; Nobel Prize; Russell Grimes; Louis Fieser; bat bomb; bats; Japan; World War II; crystal; X-ray crystallography; electron pair; Nobel Prize in Chemistry; Roald Hoffmann; Thomas Steitz; National Science Foundation; NSF; biochemistry; protein; Richard Feynman; practical joker.