
Ice Formation
August 19, 2024 In today's bigger is better world, you don't order a large coffee, you order a 20 fluid ounce Venti coffee. From 1987 through 2004, McDonald's restaurants had a supersize option for larger than large portions of its French fries and soft drinks. The demise of this supersize upselling was precipitated by the 2004 documentary, Super Size Me, by director, Morgan Spurlock (1970-2024).[1] Spurlock dined at McDonald's restaurants three times a day for thirty days, consuming an average of 5,000 kcal per day, far in excess of the recommended 2,400-2,800 kcal per day for adult men. His documentary highlighted the rise in obesity in the United States, as shown in the figure.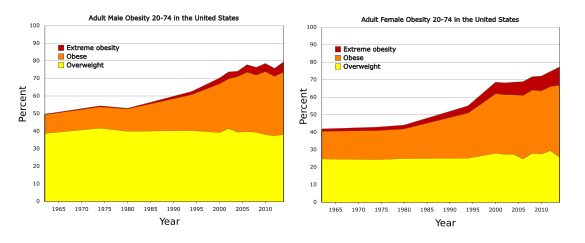
Adult obesity in the United States, 1962 to 2014, as measured by the Centers for Disease Control and Prevention (CDC). (Left image and right image by Delphi234, both from Wikimedia Commons. Click for larger image.)
The prefix, super, has been used to describe supercooling, the unexpected cooling without a phase change when liquids can be cooled below their freezing points without solidifying. I was introduced to the concept of supercooling in elementary school while listening to my shortwave radio and hearing an extended discourse on the topic by an amateur radio operator. Stream of Consciousness radio chatter didn't start with the cellphone/ It's existed on the amateur radio bands since their inception. As I wrote in an earlier article (Supercooled Water, December 22, 2011), Water has so many unusual properties that there's a website dedicated to them.[2] The unusual properties of water most probably are a result of water molecule being small, and the forces holding these molecules together in a liquid or solid arise from hydrogen bonding. There are presently twenty confirmed solid phases of water ice. The most common ice, the one that plagues our winter driving, is hexagonal ice Ih, and the others exist at different temperatures and pressures. Supercooled water is a hazard to aviation, since supercooled water droplets often existing in cumulus and stratus clouds will instantly freeze on aircraft surfaces and plug the Pitot tubes that indicate airspeed. It's easy to create supercooled water in the laboratory. You just need to purify the water to remove contained minerals. The mineral crystals act as nucleation sites, an effect behind the idea that bearded chemists have better luck with crystallizing reaction products. High purity water will only supercool to -48.3 °C (-54.9 °F). All of this molecular dynamics is reflected in thermodynamics, since the heat capacity and compressibility of supercooled water would become infinite at that temperature.[3] Ice nucleation on surfaces is the topic of a recent article in Quanta Magazine by Elise Cutts, a geobiologists who is now a science journalist in Graz, Austria. One of my graduate school classmates did his thesis experiments on the deposition of thin silver films on salt crystals (halite). The arrangement of atoms at the halite surface matches that of silver, and this facilitated the deposition of the silver films. The same is true for surfaces that nucleate ice.[4] Those whose arrangement of surface atoms mimic that of ice are good at ice nucleation.[4]
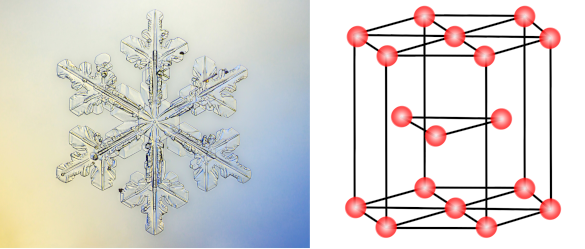
Snowflake and a Structural model of an hexagonal close packed crystal. The six-fold radial symmetry of some snowflakes arises from the hexagonal crystalline structure of ordinary ice (ice Ih). (Left, a Wikimedia Commons image by Janek Lass. Right image created using Inkscape and also uploaded to Wikimedia Commons. Click for larger image.)
Valeria Molinero and colleagues at the University of Utah (Salt Lake City, Utah) have developed a model for prediction of the nucleation temperature of ice on a given surface.[4] Model parameters include the shapes of surface defects, and appropriately sized and shaped surface bumps and depressions can squeeze water molecules into configurations that make it easier or harder for ice to form.[4] Molinero says that knowing what surface features nucleate ice will allow control of ice formation.[4] Bacteria and fungi are efficient natural ice nucleators because of the way their proteins act as ice templates.[4] The best such natural ice nucleators the Pseudomonas syringae bacterium, which is used to make artificial snow.[4] Larger protein molecules are usually better at ice nucleation, but small fungal proteins are good at ice nucleation when they clump into larger aggregates.[4] Molinero states that atmospheric models of cloud formation can be improved if the ice nucleation process is better understood.[4]
References:
- Super Size Me, 2004, Morgan Spurlock, Director, on the Internet Movie Database.
- Martin Chaplin, "Anomalous properties of water," London South Bank University Web Site.
- Emily B. Moore and Valeria Molinero, "Structural transformation in supercooled water controls the crystallization rate of ice," Nature, vol. 479, no. 7374 (November 24, 2011), pp. 506-508.
- Elise Cutts, "The Enduring Mystery of How Water Freezes." Quanta Magazine, June 17, 2024.
- Debdas Dhabal, Rajat Kumar, and Valeria Molinero, "Liquid–liquid transition and ice crystallization in a machine-learned coarse-grained water model," Proc. Natl. Acad. Sci., vol. 121, no. 20(May 6, 2024), Article no. e2322853121, https://doi.org/10.1073/pnas.2322853121.