Antimony Nanocrystals
April 7, 2014
I learned the
periodic table of the
elements as an
elementary school student, so it wasn't much of a surprise to anyone when I became a
scientist. Some of the
symbols for the elements make a lot of sense.
Helium is "He,"
oxygen is "O," and
magnesium is "Mg." Others, like the symbol for
iron, are a little strange, but
English does have words like "ferrous," so "Fe" isn't too far out of line. Then there's
antimony. The symbol for antimony is "Sb," which comes from its
Latin name, stibium.
Jöns Jakob Berzelius, a
chemist famous for so many things, used the symbol, "Sb," and it stuck.
Antimony has been known from antiquity, and a method for isolation of
metallic antimony was mentioned in
De re metallica by
Georgius Agricola in 1556. Agricola's text does not distinguish between
stibnite (antimony sulfide, Sb
2S
3), the principal
ore of antimony, and the metal, calling both "stibium." Agricola writes that the properties of antimony are like those of
lead, and he cites a "bookseller's alloy" of antimony and
tin for making
movable type for printing.[1]
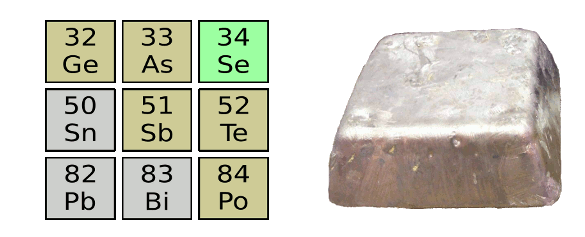
The neighbors of antimony in the periodic table. Antimony is not as toxic as arsenic, but it is chemically very similar. (Photograph of antimony ingot via Wikimedia Commons.)
Agricola's text is interesting, since its
formulations are quite exact, although written in the chemical language of the time; for example,
silver is also parted from gold by means of stibium. If in a bes of gold there are seven, or six, or five double sextulae of silver, then three parts of stibium are added to one part of gold; but in order that the stibium should not consume the gold, it is melted with copper in a red hot earthen crucible.[2]
Antimony has some modern uses, but it was not very a very useful metal in antiquity. Although it has about the same
Mohs hardness as copper, it's a
brittle material, so it's not easily
worked. It has a layered
crystal structure of ruffled, six-membered rings with
weak bonding between the layers, resulting in its brittleness and low
strength.
Small additions of antimony harden both tin and lead, and it's used in the manufacture of the lead plates of
lead-acid storage batteries where it also reduces generation of
hydrogen. One important use of antimony is as a component of the
chalcogenide glasses,
AgInSbTe and
GeSbTe. These are used as
phase change materials in
optical disks and
phase-change memory devices.
More than half of all antimony is used in the form of
antimony trioxide (Sb
2O
3) as a
flame retardant.
Indium antimonide functions as an excellent
mid-infrared photodetector for
thermal imaging with
room temperature photosensitivity up to about 7.5
μm.[3]
Scientists from
ETH Zürich (Zürich, Switzerland) and
Empa - the Swiss Federal Laboratories for Materials Science and Technology (Dübendorf, Switzerland) have recently investigated antimony
nanocrystals as an
anode material for
lithium-ion batteries.[4-5] Since there's a concern about a potential scarcity of
lithium, the research also looked at the equivalent
sodium-ion anodes.[5]
Anodes for lithium-ion batteries are presently made of
graphite, and the lithium or sodium ions are shuttled between the anode and
cathode during
charging and discharging. Lithium ions will move easily in and out of good anode and cathode materials, and the
shape and integrity of these electrodes shouldn't change as the ions enter and leave.[5]
Antimony is a good candidate for a battery electrode since it has twice the charging capacity of graphite and it will store both lithium and sodium ions.[5] Still, charging with either ion leads to a large
volume change in antimony, so the
research team investigated whether antimony in nanocrystal form might allow a more rapid volume change without
fracture. Such nanocrystals can be mixed with a
conductive carbon filler to prevent the aggregation of the nanoparticles.[5]
Monodisperse antimony nanocrystals were prepared by
colloidal synthesis with a narrow
size distribution in a tunable size range of 10-20 nm.[4] There were two reasons for this choice of size range. Small nanocrystals of 10 nm diameter and smaller have a large
surface area to volume ratio, so they're susceptible to
oxidation. Larger nanocrystals aren't small enough to resist the necessary volume expansion and contraction.[5]
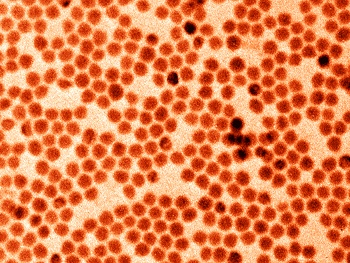
false-colored transmission electron microscope image of monodisperse antimony nanocrystals.
(Photo: Maksym Kovalenko Group / ETH Zurich.)
Experiments showed that the nanocrystal electrodes worked well for both lithium and sodium ions. The charge capacity for either ion was about 580–640
mA-h/
gram.[4] The charge capacity of the 20 nm antimony nanocrystals proved to be better than that of the 10 nm nanocrystals, and the sodium ion performance was comparable to that of the best lithium anodes.[4]
It will be another
decade before we see sodium ion batteries as a commercial product, but there's also a problem of
economics. As study co-author,
Maksym V. Kovalenko of ETH Zürich, admits,
"All in all, batteries with sodium-ions and antimony nanocrystals as anodes will only constitute a highly promising alternative to today's lithium-ion batteries if the costs of producing the batteries will be comparable."[5]
References:
- Georg Agricola, "De re metallica," Hieron Frobenium et Nicolaum Episcopium, (Basil, 1556), in Latin, via the Internet Archive.
- Herbert Clark Hoover and Lou Henry Hoover, translators, "De Re Metallica" by Georgius Agricola, Dover Publications, (New York, 1950), p. 452.
- D. G. Avery, D. W. Goodwin, W. D. Lawson and T. S. Moss, "Optical and Photo-Electrical Properties of Indium Antimonide," Proceedings of the Physical Society, vol. B67, no. 10 (October 1, 1954), pp. 761 ff.
- Meng He, Kostiantyn Kravchyk, Marc Walter and Maksym V. Kovalenko, "Monodisperse Antimony Nanocrystals for High-Rate Li-ion and Na-ion Battery Anodes: Nano versus Bulk," Nano Lett., vol. 14, no. 3 (March 12, 2014), pp 1255-1262.
- Peter Rüegg, "Antimony nanocrystals for batteries," ETH Zurich Press Release, March 18, 2014.
Permanent Link to this article
Linked Keywords: Periodic table; chemical element; elementary school; student; scientist; symbol; helium; oxygen; magnesium; iron; English language; antimony; Latin; Jöns Jakob Berzelius; chemist; metal; metallic; De re metallica; Georgius Agricola; stibnite; ore; lead; tin; movable type; arsenic; chemical reaction; chemically; ingot; Wikimedia Commons; formulation; silver; gold; copper; incandescence; red hot; clay; earthen; crucible; Mohs hardness; brittleness; brittle; material; metalworking; crystal structure; van der Waals force; weak bonding; strength of materials; lead-acid storage battery; hydrogen; chalcogenide glass; AgInSbTe; GeSbTe; phase transition; phase change; optical disk; phase-change memory device; antimony trioxide; flame retardant; Indium antimonide; mid-infrared; photodetector; thermography; thermal imaging; room temperature; micrometer; μm; ETH Zürich (Zürich, Switzerland); Empa; Swiss Federal Laboratories for Materials Science and Technology (Dübendorf, Switzerland); nanocrystal; anode; lithium-ion battery; lithium; sodium; graphite; cathode; charging and discharging; shape; volume; research; fracture mechanics; electrical conductor; conductive; carbon; dispersity; monodisperse; colloid; colloidal; synthesis; normal distribution; surface area to volume ratio; oxide; oxidation; false-colored; ransmission electron microscope; experiment; ampere-hour; mA-h; gram; decade; economics; Maksym V. Kovalenko.